当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exosomes from Irradiated Nonsmall Cell Lung Cancer Cells Reduced Sensitivity of Recipient Cells to Anaplastic Lymphoma Kinase Inhibitors
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-03-29 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00059 Hao Wu 1 , Chao Zeng 2 , Yiwang Ye 1 , Jixian Liu 1 , Zhimin Mu 1 , Yuancai Xie 1 , Baokun Chen 1 , Qiaohong Nong 3 , Da Wu 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-03-29 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00059 Hao Wu 1 , Chao Zeng 2 , Yiwang Ye 1 , Jixian Liu 1 , Zhimin Mu 1 , Yuancai Xie 1 , Baokun Chen 1 , Qiaohong Nong 3 , Da Wu 1
Affiliation
|
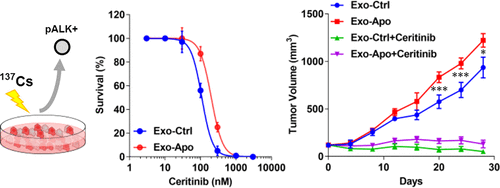
Exosomes, released from various cell types, serve as vehicles of intercellular communication. Rearranged anaplastic lymphoma kinase (ALK) has been detected in exosomes released from cancer cells in ALK-positive nonsmall cell lung cancer (NSCLC), however, the functional consequence of ALK in exosomes has not been studied. This study aims to address whether exosomal ALK release is affected by stress, and whether exosomal ALK can modulate survival of recipient cells in vitro and in vivo. Exosomes, isolated from ALK-containing H3122 cells with (Exo-Apo) or without (Exo-Ctrl) irradiation treatment, were transferred to recipient H3122 cells in vitro or mouse xenograft in vivo. Western blot, flow cytometry, MTT, and xenograft were employed to respectively assess activation of the ALK pathway, apoptosis, cell viability, and tumor growth. Exo-Apo contained much higher levels of phosphorylated ALK (p-ALK) than that of Exo-Ctrl, and it activated AKT, STAT3, and the ERK pathway in recipient H3122 cells. ALK-specific inhibitors, including Crizotinib, Ceritinib, and TAE684, exhibited less effects on H3122 cells preincubated with Exo-Apo than on those treated with Exo-Ctrl in either inhibition of cell viability or promotion of apoptosis. Moreover, in an H3122 xenograft model, the Exo-Apo treatment resulted in a greater tumor growth and less sensitivity to Ceritinib than the Exo-Ctrl treatment. The ALK protein cargo in exosomes could be a key element to drive tumor growth and compromise therapeutic efficacy of ALK inhibitors for ALK-positive NSCLC.
中文翻译:

辐射非小细胞肺癌细胞的外来体降低了收件人细胞对间变性淋巴瘤激酶抑制剂的敏感性。
从各种细胞类型中释放出来的外来体充当细胞间通讯的载体。已在ALK阳性非小细胞肺癌(NSCLC)的癌细胞释放的外泌体中检测到重排的间变性间变性淋巴瘤激酶(ALK),但尚未研究ALK在外泌体中的功能后果。这项研究旨在解决外泌体ALK释放是否受到压力的影响,以及外泌体ALK是否可以调节体外和体内受体细胞的存活。从含ALK的H3122细胞(经(Exo-Apo)或未经(Exo-Ctrl)照射处理)中分离出的外泌体,在体外或体内异种移植后被转移至受体H3122细胞。使用蛋白质印迹,流式细胞术,MTT和异种移植分别评估ALK途径的激活,凋亡,细胞生存力和肿瘤生长。Exo-Apo的磷酸化ALK(p-ALK)含量比Exo-Ctrl高得多,并且它激活了受体H3122细胞中的AKT,STAT3和ERK途径。ALK特异性抑制剂(包括克唑替尼,Ceritinib和TAE684)对用Exo-Apo预孵育的H3122细胞的抑制作用比用Exo-Ctrl处理的抑制剂抑制细胞活力或促进细胞凋亡的作用要小。此外,在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。受体H3122细胞中的ERK途径。ALK特异性抑制剂(包括克唑替尼,Ceritinib和TAE684)对用Exo-Apo预孵育的H3122细胞的抑制作用比用Exo-Ctrl处理的抑制剂抑制细胞活力或促进细胞凋亡的作用要小。此外,在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。受体H3122细胞中的ERK途径。ALK特异性抑制剂(包括克唑替尼,Ceritinib和TAE684)对用Exo-Apo预孵育的H3122细胞的抑制作用比用Exo-Ctrl处理的抑制剂抑制细胞活力或促进细胞凋亡的作用要小。此外,在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。
更新日期:2018-03-29
中文翻译:

辐射非小细胞肺癌细胞的外来体降低了收件人细胞对间变性淋巴瘤激酶抑制剂的敏感性。
从各种细胞类型中释放出来的外来体充当细胞间通讯的载体。已在ALK阳性非小细胞肺癌(NSCLC)的癌细胞释放的外泌体中检测到重排的间变性间变性淋巴瘤激酶(ALK),但尚未研究ALK在外泌体中的功能后果。这项研究旨在解决外泌体ALK释放是否受到压力的影响,以及外泌体ALK是否可以调节体外和体内受体细胞的存活。从含ALK的H3122细胞(经(Exo-Apo)或未经(Exo-Ctrl)照射处理)中分离出的外泌体,在体外或体内异种移植后被转移至受体H3122细胞。使用蛋白质印迹,流式细胞术,MTT和异种移植分别评估ALK途径的激活,凋亡,细胞生存力和肿瘤生长。Exo-Apo的磷酸化ALK(p-ALK)含量比Exo-Ctrl高得多,并且它激活了受体H3122细胞中的AKT,STAT3和ERK途径。ALK特异性抑制剂(包括克唑替尼,Ceritinib和TAE684)对用Exo-Apo预孵育的H3122细胞的抑制作用比用Exo-Ctrl处理的抑制剂抑制细胞活力或促进细胞凋亡的作用要小。此外,在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。受体H3122细胞中的ERK途径。ALK特异性抑制剂(包括克唑替尼,Ceritinib和TAE684)对用Exo-Apo预孵育的H3122细胞的抑制作用比用Exo-Ctrl处理的抑制剂抑制细胞活力或促进细胞凋亡的作用要小。此外,在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。受体H3122细胞中的ERK途径。ALK特异性抑制剂(包括克唑替尼,Ceritinib和TAE684)对用Exo-Apo预孵育的H3122细胞的抑制作用比用Exo-Ctrl处理的抑制剂抑制细胞活力或促进细胞凋亡的作用要小。此外,在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。在H3122异种移植模型中,与Exo-Ctrl处理相比,Exo-Apo处理导致更大的肿瘤生长和对Ceritinib的敏感性降低。外泌体中的ALK蛋白可能是驱动肿瘤生长并损害ALK抑制剂对ALK阳性NSCLC的治疗功效的关键因素。


























 京公网安备 11010802027423号
京公网安备 11010802027423号