当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering an [FeFe]-hydrogenase: do accessory clusters influence O2 resistance and catalytic bias?
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-03-29 , DOI: 10.1021/jacs.8b01689 Giorgio Caserta 1 , Cecilia Papini 1 , Agnieszka Adamska-Venkatesh 2 , Ludovic Pecqueur 1 , Constanze Sommer 2 , Edward Reijerse 2 , Wolfgang Lubitz 2 , Charles Gauquelin 3 , Isabelle Meynial-Salles 3 , Debajyoti Pramanik 4 , Vincent Artero 4 , Mohamed Atta 4 , Melisa del Barrio 5 , Bruno Faivre 1 , Vincent Fourmond 5 , Christophe Léger 5 , Marc Fontecave 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-03-29 , DOI: 10.1021/jacs.8b01689 Giorgio Caserta 1 , Cecilia Papini 1 , Agnieszka Adamska-Venkatesh 2 , Ludovic Pecqueur 1 , Constanze Sommer 2 , Edward Reijerse 2 , Wolfgang Lubitz 2 , Charles Gauquelin 3 , Isabelle Meynial-Salles 3 , Debajyoti Pramanik 4 , Vincent Artero 4 , Mohamed Atta 4 , Melisa del Barrio 5 , Bruno Faivre 1 , Vincent Fourmond 5 , Christophe Léger 5 , Marc Fontecave 1
Affiliation
|
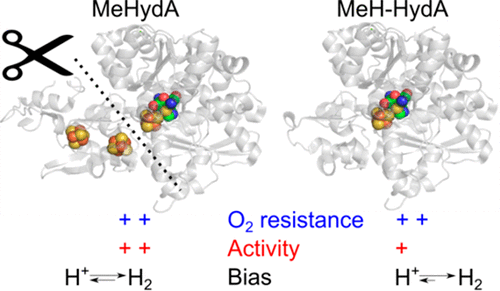
[FeFe]-hydrogenases, HydAs, are unique biocatalysts for proton reduction to H2. However, they suffer from a number of drawbacks for biotechnological applications: size, number and diversity of metal cofactors, oxygen sensitivity. Here we show that HydA from Megasphaera elsdenii (MeHydA) displays significant resistance to O2. Furthermore, we produced a shorter version of this enzyme (MeH-HydA), lacking the N-terminal domain harboring the accessory FeS clusters. As shown by detailed spectroscopic and biochemical characterization, MeH-HydA displays the following interesting properties. First, a functional active site can be assembled in MeH-HydA in vitro, providing the enzyme with excellent hydrogenase activity. Second, the resistance of MeHydA to O2 is conserved in MeH-HydA. Third, MeH-HydA is more biased toward proton reduction than MeHydA, as the result of the truncation changing the rate limiting steps in catalysis. This work shows that it is possible to engineer HydA to generate an active hydrogenase that combines the resistance of the most resistant HydAs and the simplicity of algal HydAs, containing only the H-cluster.
中文翻译:

设计 [FeFe]-氢化酶:辅助簇会影响 O2 电阻和催化偏差吗?
[FeFe]-氢化酶 HydAs 是用于质子还原为 H2 的独特生物催化剂。然而,它们在生物技术应用方面存在许多缺点:金属辅因子的大小、数量和多样性、氧敏感性。在这里,我们展示了来自 Megasphaera elsdenii (MeHydA) 的 HydA 对 O2 显示出显着的抗性。此外,我们生产了这种酶的较短版本(MeH-HydA),缺乏包含辅助 FeS 簇的 N 端结构域。正如详细的光谱和生化表征所示,MeH-HydA 显示出以下有趣的特性。首先,功能性活性位点可以在体外组装在 MeH-HydA 中,为酶提供优异的氢化酶活性。其次,MeHydA 对 O2 的抗性在 MeH-HydA 中是保守的。第三,MeH-HydA 比 MeHydA 更偏向质子还原,由于截断改变了催化中的限速步骤。这项工作表明,可以设计 HydA 以产生活性氢化酶,该酶结合了最具抗性的 HydA 的抗性和藻类 HydAs 的简单性,仅包含 H 簇。
更新日期:2018-03-29
中文翻译:

设计 [FeFe]-氢化酶:辅助簇会影响 O2 电阻和催化偏差吗?
[FeFe]-氢化酶 HydAs 是用于质子还原为 H2 的独特生物催化剂。然而,它们在生物技术应用方面存在许多缺点:金属辅因子的大小、数量和多样性、氧敏感性。在这里,我们展示了来自 Megasphaera elsdenii (MeHydA) 的 HydA 对 O2 显示出显着的抗性。此外,我们生产了这种酶的较短版本(MeH-HydA),缺乏包含辅助 FeS 簇的 N 端结构域。正如详细的光谱和生化表征所示,MeH-HydA 显示出以下有趣的特性。首先,功能性活性位点可以在体外组装在 MeH-HydA 中,为酶提供优异的氢化酶活性。其次,MeHydA 对 O2 的抗性在 MeH-HydA 中是保守的。第三,MeH-HydA 比 MeHydA 更偏向质子还原,由于截断改变了催化中的限速步骤。这项工作表明,可以设计 HydA 以产生活性氢化酶,该酶结合了最具抗性的 HydA 的抗性和藻类 HydAs 的简单性,仅包含 H 簇。


























 京公网安备 11010802027423号
京公网安备 11010802027423号