Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-03-28 , DOI: 10.1016/j.tetlet.2018.03.084 Shimaa A.H. Abdel Monaim , Gerardo A. Acosta , Miriam Royo , Ayman El-Faham , Beatriz G. de la Torre , Fernando Albericio
|
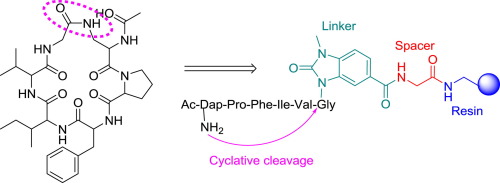
Cyclic homodetic peptides are very appealing for medicinal chemistry programs. In addition to the high efficiency and selectivity inherently associated with peptides, a cyclic structure totally formed by amide bonds increases their stability under physiological conditions. Here Fmoc-MeDbz-resin was studied for the preparation of these peptides. Our results demonstrate the usefulness of this strategy for the preparation of cyclic “head-to-side chain” peptides through cyclative cleavage (simultaneous cyclization and release from the resin). In contrast, for the synthesis of the “head-to-tail” counterparts, the cyclization-cleavage should be carried out in the presence of thiophenol.
中文翻译:

Fmoc-MeDbz-树脂固相合成同源环状肽
环状同源肽对于药物化学程序非常有吸引力。除了与肽固有的高效率和选择性外,完全由酰胺键形成的环状结构还提高了它们在生理条件下的稳定性。在这里,研究了Fmoc-MeDbz-树脂用于制备这些肽。我们的结果证明了该策略通过循环裂解(同时环化和从树脂中释放)制备环状“头对侧链”肽的有用性。相反,为了合成“从头到尾”的对应物,环化裂解应在硫酚存在下进行。


























 京公网安备 11010802027423号
京公网安备 11010802027423号