当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
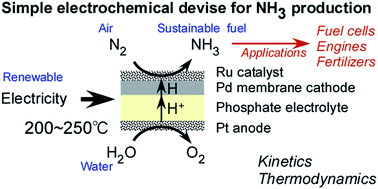
Electrochemical membrane cell for NH3 synthesis from N2 and H2O by electrolysis at 200 to 250 °C using a Ru catalyst, hydrogen-permeable Pd membrane and phosphate-based electrolyte
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2018-03-28 , DOI: 10.1039/c8se00054a Kanako Imamura 1, 2, 3, 4 , Jun Kubota 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2018-03-28 , DOI: 10.1039/c8se00054a Kanako Imamura 1, 2, 3, 4 , Jun Kubota 1, 2, 3, 4
Affiliation
|
Ammonia (NH3) is an energy carrier that can be synthesized from nitrogen and water using electricity generated from renewable sources. The present work investigated NH3 synthesis using an electrochemical system with the structure of Ru/Cs+/MgO|Pd–Ag|CsH2PO4/SiP2O7|Pt and operating at 200 to 250 °C. In this system, the NH3 being generated is isolated from the CsH2PO4/SiP2O7 electrolyte by the hydrogen-permeable Pd–Ag membrane, resulting in the production of dry NH3. A maximum NH3 synthesis rate of 0.90 nmol s−1 cm−2 was obtained from the cathode at the Ru/Cs+/MgO|Pd–Ag side at a current density of 10 mA cm−2, temperature of 250 °C and N2 flow rate of 3 cm3 min−1 as converted to standard temperature and pressure. Deviations in the NH3 production rate from the theoretical amounts predicted by Arrhenius plots were observed at temperatures approaching 250 °C, possibly because the reaction approached equilibrium. A current efficiency for NH3 production of 2.6% was obtained, with the remainder of the current consumed during H2 generation. The apparent activation energies for the NH3 synthesis were 69 and 93 kJ mol−1 at 3.2 and 10 mA cm−2, respectively. These values are significantly lower than that reported for conventional NH3 synthesis from nitrogen and hydrogen (139 kJ mol−1) over the same catalyst in this cell. The optimum N2 flow rates were approximately 0.5 and 5 cm3 min−1 at 3.2 and 10 mA cm−2, respectively, representing nitrogen amounts approximately 42 times the stoichiometric quantities. The provision of an excess of N2 is believed to reduce the suppression of N2 activation by so-called hydrogen poisoning of the catalyst.
中文翻译:

使用Ru催化剂,氢可渗透的Pd膜和磷酸盐基电解质在200至250°C的温度下通过电解从N 2和H 2 O合成NH 3的电化学膜电池
氨(NH 3)是一种能源载体,可以使用可再生资源产生的电能由氮和水合成。本工作研究了使用Ru / Cs + / MgO | Pd–Ag | CsH 2 PO 4 / SiP 2 O 7 | Pt结构的电化学系统在200至250°C的温度下合成NH 3的过程。在该系统中,通过氢可渗透的Pd-Ag膜从CsH 2 PO 4 / SiP 2 O 7电解质中分离出正在生成的NH 3,从而产生干燥的NH 3。最高NH 3在10 mA cm -2的电流密度,250°C的温度和N 2流速下,从Ru / Cs + / MgO | Pd-Ag侧的阴极获得0.90 nmol s -1 cm -2的合成速率3 cm 3 min -1转换为标准温度和压力。在接近250°C的温度下,观察到NH 3生成速率与Arrhenius图所预测的理论量存在偏差,这可能是因为反应已趋于平衡。NH 3生产的电流效率为2.6%,其余电流在H 2期间消耗一代。NH 3合成的表观活化能在3.2和10mA cm -2分别为69和93kJ mol -1。这些值明显低于在该电池中的相同催化剂上由氮气和氢气(139 kJ mol -1)合成常规NH 3的报告值。最佳N 2流速分别为3.2和10 mA cm -2时分别约为0.5和5 cm 3 min -1,代表氮含量约为化学计量的42倍。认为提供过量的N 2减少了对N 2的抑制。 通过所谓的催化剂氢中毒而活化。
更新日期:2018-05-30
中文翻译:

使用Ru催化剂,氢可渗透的Pd膜和磷酸盐基电解质在200至250°C的温度下通过电解从N 2和H 2 O合成NH 3的电化学膜电池
氨(NH 3)是一种能源载体,可以使用可再生资源产生的电能由氮和水合成。本工作研究了使用Ru / Cs + / MgO | Pd–Ag | CsH 2 PO 4 / SiP 2 O 7 | Pt结构的电化学系统在200至250°C的温度下合成NH 3的过程。在该系统中,通过氢可渗透的Pd-Ag膜从CsH 2 PO 4 / SiP 2 O 7电解质中分离出正在生成的NH 3,从而产生干燥的NH 3。最高NH 3在10 mA cm -2的电流密度,250°C的温度和N 2流速下,从Ru / Cs + / MgO | Pd-Ag侧的阴极获得0.90 nmol s -1 cm -2的合成速率3 cm 3 min -1转换为标准温度和压力。在接近250°C的温度下,观察到NH 3生成速率与Arrhenius图所预测的理论量存在偏差,这可能是因为反应已趋于平衡。NH 3生产的电流效率为2.6%,其余电流在H 2期间消耗一代。NH 3合成的表观活化能在3.2和10mA cm -2分别为69和93kJ mol -1。这些值明显低于在该电池中的相同催化剂上由氮气和氢气(139 kJ mol -1)合成常规NH 3的报告值。最佳N 2流速分别为3.2和10 mA cm -2时分别约为0.5和5 cm 3 min -1,代表氮含量约为化学计量的42倍。认为提供过量的N 2减少了对N 2的抑制。 通过所谓的催化剂氢中毒而活化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号