当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Towards oxalate-bridged iron(ii), cobalt(ii), nickel(ii) and zinc(ii) complexes through oxotris(oxalato)niobate(v): an open air non-oxidizing synthetic route†
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2018-03-28 00:00:00 , DOI: 10.1039/c8qi00191j Willian X. C. Oliveira 1, 2, 3, 4 , Cynthia L. M. Pereira 1, 2, 3, 4 , Carlos B. Pinheiro 2, 3, 4, 5 , Francesc Lloret 6, 7, 8, 9 , Miguel Julve 6, 7, 8, 9
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2018-03-28 00:00:00 , DOI: 10.1039/c8qi00191j Willian X. C. Oliveira 1, 2, 3, 4 , Cynthia L. M. Pereira 1, 2, 3, 4 , Carlos B. Pinheiro 2, 3, 4, 5 , Francesc Lloret 6, 7, 8, 9 , Miguel Julve 6, 7, 8, 9
Affiliation
|
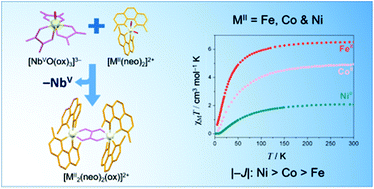
Four compounds with the formula [M2(dmphen)4(μ-C2O4)](ClO4)2·2dmso [M = Fe (1), Co (2) and Zn (4); dmphen = 2,9-dimethyl-1,10-phenanthroline] and [Ni2(dmphen)4(μ-C2O4)]3[NbO(C2O4)3]2·16H2O (3) have been synthesized using the tris(oxalato)oxoniobate(V) complex anion as the oxalate source, and their structures have been determined by single crystal X-ray diffraction. X-ray quality crystals of highly insoluble oxalate-bridged species were obtained by taking advantage of the slow release of oxalate by the tris(oxalato)oxoniobate(V) complex anion. The structures of 1–4 all contain oxalate-bridged dimetal(II) units with didentate dmphen molecules acting as end-cap ligands; electroneutrality is achieved by perchlorate (1–4) and oxotris(oxalato)niobate(V) (3) anions. Each divalent metal ion in 1–4 is tris-chelated in a six-coordinate distorted octahedral environment. The niobium(V) ion in 3 is seven-coordinate in a distorted pentagonal bipyramidal geometry built from one oxo group and six oxygen atoms from three didentate oxalate ligands. The values of the metal–metal separation across the bis-chelating oxalate are 5.626(1) (1), 5.575(1) (2), 5.434(1) to 5.447(1) (3) and 5.603(1) Å (4). The cryomagnetic measurements in the temperature range of 2.0 to 300 K for compounds 1–3 show the occurrence of antiferromagnetic interactions between the divalent metal ions across the oxalate bridge. The nature and amplitude of these magnetic interactions are rationalized by simple symmetry considerations and compared with those previously reported for related oxalate-bridged systems.
中文翻译:

通过草酸氧杂草酸酯(v)向草酸盐桥联的铁(ii),钴(ii),镍(ii)和锌(ii)络合物过渡:一种露天的非氧化性合成途径†
四种化合物与式[M 2(dmphen)4(μ-C 2 Ò 4)](CLO 4)2 ·2dmso [M = Fe(上1),CO(2)和Zn(4); dmphen = 2,9-二甲基-1,10-菲咯啉]和[镍2(dmphen)4(μ-C 2 Ò 4)] 3 [NbO的(C 2 Ò 4)3 ] 2 ·16H 2 O(3)用三(草酸)氧杂酸酯(V阴离子作为草酸盐源,其结构已通过单晶X射线衍射确定。通过利用三(草酸酯)氧杂酸根(V)络合物阴离子缓慢释放草酸根,获得了高度不溶的草酸桥连物种的X射线质量晶体。1–4的结构均包含草酸盐桥联的双金属(II)单元,其中二齿dmphen分子充当封端配体。电子高中性是通过高氯酸根(1-4)和氧杂三酸(草酸)铌酸根(V)(3)阴离子实现的。1-4中的每个二价金属离子在六坐标扭曲的八面体环境中被三螯合。铌(V)3中的离子在由一个氧代基团和三个三齿草酸酯配体组成的六个氧原子构成的扭曲的五边形双锥体几何结构中为七坐标。双螯合草酸盐的金属-金属分离值为5.626(1)(1),5.575(1)(2),5.434(1)至5.447(1)(3)和5.603(1)Å(4)。对于化合物1-3,在2.0至300 K的温度范围内进行低温磁测量图2显示了横跨草酸盐桥的二价金属离子之间反铁磁相互作用的发生。这些磁性相互作用的性质和幅度可通过简单的对称性考虑加以合理化,并与先前报道的有关草酸盐桥联体系的那些相互作用进行比较。
更新日期:2018-03-28
中文翻译:

通过草酸氧杂草酸酯(v)向草酸盐桥联的铁(ii),钴(ii),镍(ii)和锌(ii)络合物过渡:一种露天的非氧化性合成途径†
四种化合物与式[M 2(dmphen)4(μ-C 2 Ò 4)](CLO 4)2 ·2dmso [M = Fe(上1),CO(2)和Zn(4); dmphen = 2,9-二甲基-1,10-菲咯啉]和[镍2(dmphen)4(μ-C 2 Ò 4)] 3 [NbO的(C 2 Ò 4)3 ] 2 ·16H 2 O(3)用三(草酸)氧杂酸酯(V阴离子作为草酸盐源,其结构已通过单晶X射线衍射确定。通过利用三(草酸酯)氧杂酸根(V)络合物阴离子缓慢释放草酸根,获得了高度不溶的草酸桥连物种的X射线质量晶体。1–4的结构均包含草酸盐桥联的双金属(II)单元,其中二齿dmphen分子充当封端配体。电子高中性是通过高氯酸根(1-4)和氧杂三酸(草酸)铌酸根(V)(3)阴离子实现的。1-4中的每个二价金属离子在六坐标扭曲的八面体环境中被三螯合。铌(V)3中的离子在由一个氧代基团和三个三齿草酸酯配体组成的六个氧原子构成的扭曲的五边形双锥体几何结构中为七坐标。双螯合草酸盐的金属-金属分离值为5.626(1)(1),5.575(1)(2),5.434(1)至5.447(1)(3)和5.603(1)Å(4)。对于化合物1-3,在2.0至300 K的温度范围内进行低温磁测量图2显示了横跨草酸盐桥的二价金属离子之间反铁磁相互作用的发生。这些磁性相互作用的性质和幅度可通过简单的对称性考虑加以合理化,并与先前报道的有关草酸盐桥联体系的那些相互作用进行比较。


























 京公网安备 11010802027423号
京公网安备 11010802027423号