Dyes and Pigments ( IF 4.5 ) Pub Date : 2018-03-27 , DOI: 10.1016/j.dyepig.2018.03.056 Robby Vroemans , My Thi Dieu Tran , Mian Gul Sayed , Stijn Boodts , Wim Dehaen
|
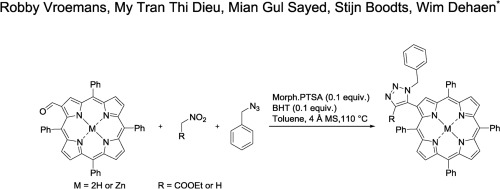
The regioselective synthesis of novel 1,5- and 1,4,5-substituted 1,2,3-triazoles linked to the β-position of 5,10,15,20-tetraphenylporphyrins and their zincated derivatives were achieved via an organocatalytic multicomponent reaction, starting from easily available β-formyl 5,10,15,20-tetraphenylporphyrin, nitroalkanes and benzyl azide, in moderate yields. The properties of these new β-linked 1,2,3-triazolyl porphyrins were investigated and atropoisomerism was observed. The rotational stability of these β-linked triazolylporphyrins, deduced by 1H NMR, was calculated and all newly synthesized compounds were further characterized by UV-VIS spectroscopy and high resolution mass spectroscopy.
中文翻译:

新型轴向手性β-连接的1,2,3-三唑基卟啉的合成与表征
通过5,10,15,20-四苯基卟啉及其锌化衍生物的β-位连接的新型1,5-和1,4,5-取代的1,2,3-三唑的区域选择性合成是通过有机催化多组分实现的从容易获得的β-甲酰基5,10,15,20-四苯基卟啉,硝基烷和叠氮化苄开始进行中等程度的反应。研究了这些新的β-连接的1,2,3-三唑基卟啉的性质,并观察到阻风异构。这些β-连接的三唑基卟啉的旋转稳定性由1推导得出计算了1 H NMR,并通过UV-VIS光谱和高分辨率质谱进一步表征了所有新合成的化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号