Drug Discovery Today ( IF 7.4 ) Pub Date : 2018-03-27 , DOI: 10.1016/j.drudis.2018.03.012 Ildikó Csóka , Edina Pallagi , Tamás L. Paál
|
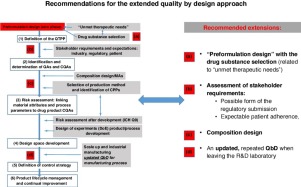
Here, we propose the extension of the quality-by-design (QbD) concept to also fit the early development phases of pharmaceuticals by adding elements that are currently widely applied, but not yet included in the QbD model in a structured way. These are the introduction of a ‘zero’ preformulation phase (i.e., selection of drug substance, possible dosage forms and administration routes based on the evaluated therapeutic need); building in stakeholders’ (industry, patient, and regulatory) requirements into the quality target product profile (QTTP); and the use of modern quality management tools during the composition and process design phase [collecting critical quality attributes (CQAs) and selection of CPPs) for (still laboratory-scale) design space (DS) development. Moreover, during industrial scale-up, CQAs (as well as critical process parameters; CPPs) can be changed; however, we recommend that the existing QbD elements are reconsidered and updated after this phase.
中文翻译:

将“按质量设计”概念扩展到药物研发过程的早期开发阶段
在这里,我们建议扩展按设计质量(QbD)概念,使其也适合于药物的早期开发阶段,方法是添加当前被广泛应用但尚未以结构化方式包含在QbD模型中的元素。这些是“零”预配制阶段的引入(即,根据评估的治疗需要选择药物,可能的剂型和给药途径);将利益相关者(行业,患者和法规)的要求纳入质量目标产品资料(QTTP);以及在组成和过程设计阶段中使用现代质量管理工具[收集关键质量属性(CQA)和选择CPP)以进行(实验室规模的)设计空间(DS)开发。此外,在工业规模放大期间,CQA(以及关键的工艺参数;CPPs)可以更改;但是,我们建议在此阶段之后重新考虑并更新现有的QbD元素。



























 京公网安备 11010802027423号
京公网安备 11010802027423号