Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2018-03-26 , DOI: 10.1016/j.jfluchem.2018.03.010 Tatsuo Miyazaki , Isamu Mori , Tomonori Umezaki , Susumu Yonezawa
|
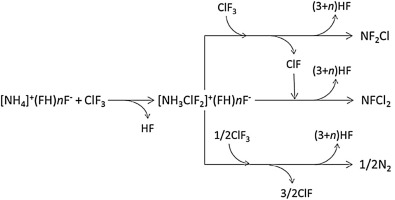
Reactions between NH4F/nHF and gaseous ClF3 were examined at temperatures of 280–320 K under various n-values (n = 2.2, 2.3, 2.4, 2.7, 2.8, 3.0, 3.1, and 4.0) at which NH4F/nHF was a molten salt, with three ClF3 gas flow rates (20, 50 and 100 standard cc min−1). From a direct reaction, NF2Cl and NFCl2 gases were obtained as products. With high NF2Cl and NFCl2 selectivity for N2, such as higher than 90% selectivity as the nitrogen containing molecule base at temperatures below 298 K, an n value of around 2.2–2.3 was obtained. The selectivity of NF2Cl and NFCl2 decreased to less than 70% at temperatures higher than 318 K because of increased amounts of N2 by-product. High selectivity of NF2Cl and NFCl2 were obtainable with the reaction mechanism in which [NH3ClF2]+(FH)nF- is formed as an intermediate.
中文翻译:

用NH 4 F / n HF和ClF 3合成NF 2 Cl和NFCl 2
NH之间的反应4 F / Ñ HF和气态的ClF 3在各种在280-320的K的温度下进行了检查ñ -值(Ñ 在该NH = 2.2,2.3,2.4,2.7,2.8,3.0,3.1和4.0)4 F / n HF是一种熔融盐,具有三种ClF 3气体流速(20、50和100标准cc min -1)。通过直接反应,获得了作为产物的NF 2 Cl和NFCl 2气体。NF 2 Cl和NFCl 2对N 2的选择性高例如,在低于298 K的温度下,作为含氮分子碱的选择性高于90%时,n值约为2.2–2.3。在高于318 K的温度下,由于N 2副产物的量增加,NF 2 Cl和NFCl 2的选择性降至70%以下。通过以[NH 3 ClF 2 ] +(FH)n F-作为中间体形成的反应机理,可以得到高选择性的NF 2 Cl和NFCl 2。



























 京公网安备 11010802027423号
京公网安备 11010802027423号