当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Expanding the Schulze–Hardy Rule and the Hofmeister Series to Nanometer‐Scaled Hydrophilic Macroions
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-03-26 , DOI: 10.1002/chem.201706101 Yang Chu 1 , Jiahui Chen 1 , Fadi Haso 1 , Yunyi Gao 1 , Jennifer E. S. Szymanowski 2 , Peter C. Burns 2 , Tianbo Liu 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-03-26 , DOI: 10.1002/chem.201706101 Yang Chu 1 , Jiahui Chen 1 , Fadi Haso 1 , Yunyi Gao 1 , Jennifer E. S. Szymanowski 2 , Peter C. Burns 2 , Tianbo Liu 1
Affiliation
|
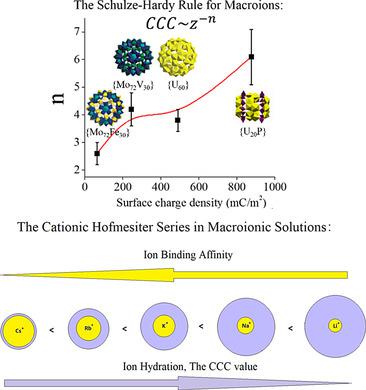
The Schulze–Hardy rule is a well‐established observation in colloid science (can be derived from the DLVO theory) that demonstrates the relationship between the critical coagulation concentration (CCC) of colloids and the valence of extra counterionic electrolytes (z), with a simple mathematical relationship of CCC≈z−6. Here the Schulze–Hardy Rule is expanded to much smaller, nano‐scaled soluble macroions in aqueous solution, by examining the stability of the macroions in the presence of additional electrolytes. The CCC values of the macroions follow the general trend of CCC≈z−n but the n value is significantly dependent on the surface charge density of the macroions, ranging from n=2 at very low surface charge density to n=6 at a high surface charge density. In addition, different cations with the same valence showed clear different impacts on the CCC values, with an interesting trend being connected to the Hofmeister series originally discovered in protein solutions.
中文翻译:

将Schulze-Hardy规则和Hofmeister系列扩展为纳米尺度的亲水性巨分子
Schulze-Hardy法则是胶体科学中一个公认的观察结果(可从DLVO理论得出),它证明了胶体的临界凝结浓度(CCC)与额外的反离子电解质(z)的化合价之间的关系。CCC≈的简单的数学关系ž -6。在这里,通过检查存在额外电解质的情况下巨离子的稳定性,将舒尔兹-哈迪法则扩展为水溶液中更小,纳米级的可溶性巨离子。所述macroions的CCC值遵循CCC≈的总趋势ž - ñ但Ñ值是显著取决于macroions的表面电荷密度,范围从Ñ在非常低的表面电荷密度下为= 2,而在很高的表面电荷密度下为n = 6。此外,具有相同价态的不同阳离子对CCC值显示出明显不同的影响,有趣的趋势与最初在蛋白质溶液中发现的Hofmeister系列有关。
更新日期:2018-03-26
中文翻译:

将Schulze-Hardy规则和Hofmeister系列扩展为纳米尺度的亲水性巨分子
Schulze-Hardy法则是胶体科学中一个公认的观察结果(可从DLVO理论得出),它证明了胶体的临界凝结浓度(CCC)与额外的反离子电解质(z)的化合价之间的关系。CCC≈的简单的数学关系ž -6。在这里,通过检查存在额外电解质的情况下巨离子的稳定性,将舒尔兹-哈迪法则扩展为水溶液中更小,纳米级的可溶性巨离子。所述macroions的CCC值遵循CCC≈的总趋势ž - ñ但Ñ值是显著取决于macroions的表面电荷密度,范围从Ñ在非常低的表面电荷密度下为= 2,而在很高的表面电荷密度下为n = 6。此外,具有相同价态的不同阳离子对CCC值显示出明显不同的影响,有趣的趋势与最初在蛋白质溶液中发现的Hofmeister系列有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号