当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sorption of Pb(II) by Nanosized Ferrihydrite Organo-Mineral Composites Formed by Adsorption versus Coprecipitation
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00005 Huihui Du 1, 2, 3, 4 , Qiaoyun Huang 2 , Ming Lei 1, 3, 4 , Boqing Tie 1, 3, 4
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00005 Huihui Du 1, 2, 3, 4 , Qiaoyun Huang 2 , Ming Lei 1, 3, 4 , Boqing Tie 1, 3, 4
Affiliation
|
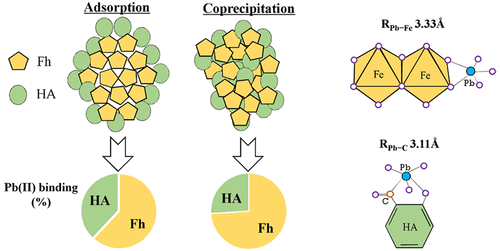
Nanosized iron hydroxides and organic matter (OM) are important scavengers of nutrient elements and dissolved trace-metals in geologic systems. Despite this, little is known about the sorption characteristics of metals to the iron–OM nanocomposites formed by adsorption (adsorption of OM on presynthesis ferrihydrite) versus coprecipitation (formation of ferrihydrite in the presence of OM). This study investigates lead (Pb) sorption behaviors on ferrihydrite–humic acid (Fh–HA) composites through batch sorption coupled with Pb-LIII EXAFS. We report that composites formed by coprecipitation have a higher carbon content, smaller specific surface area, and faster Pb sorption rate compared to the equivalent composites formed by adsorption of HA to preformed Fh. Pb sorption on the composites is enhanced at pH < 5 than that on pure Fh, and coprecipitated and adsorbed composites sorb almost equivalent amount of Pb. We identify a bidentate edge-sharing Pb complex on the ferrihydrite surface with a Pb—Fe bond length of 3.33 Å and a bidentate inner-sphere Pb complex on the HA fraction of the composite. More Pb ions are associated with the HA fraction of the composites with the increases of pH from 4 to 6.5. More Pb ions are sequestrated by the organic fraction in the adsorbed than in the coprecipitated composites. This research therefore has important implications for predicting the mobility and fate of Pb in iron and organic rich soils and sediments.
中文翻译:

纳米水铁矿有机矿物复合材料吸附与共沉淀形成的对Pb(II)的吸附
纳米氢氧化铁和有机物(OM)是地质系统中营养元素和溶解的痕量金属的重要清除剂。尽管如此,关于金属对铁-OM纳米复合材料的吸附特性知之甚少,该复合物是通过吸附(OM在预合成亚铁酸盐上的吸附)与共沉淀(在OM存在下形成亚铁酸盐)形成的。本研究通过分批吸附与Pb-L III一起研究了水铁矿-腐植酸(Fh-HA)复合材料中铅(Pb)的吸附行为EXAFS。我们报道通过共沉淀形成的复合材料与通过将HA吸附到预制Fh上形成的同等复合材料相比,具有更高的碳含量,更小的比表面积和更快的Pb吸附速率。与纯Fh相比,pH <5时,复合材料上的Pb吸附增强,并且共沉淀和吸附的复合材料吸附几乎等量的Pb。我们确定了在水铁矿表面上具有Pb-Fe键长为3.33Å的双齿边共享Pb络合物和在复合材料的HA组分上的双齿内球Pb络合物。随着pH值从4增加到6.5,更多的Pb离子与复合材料的HA组分相关。与共沉淀的复合材料相比,被吸附的有机部分螯合了更多的Pb离子。
更新日期:2018-03-26
中文翻译:

纳米水铁矿有机矿物复合材料吸附与共沉淀形成的对Pb(II)的吸附
纳米氢氧化铁和有机物(OM)是地质系统中营养元素和溶解的痕量金属的重要清除剂。尽管如此,关于金属对铁-OM纳米复合材料的吸附特性知之甚少,该复合物是通过吸附(OM在预合成亚铁酸盐上的吸附)与共沉淀(在OM存在下形成亚铁酸盐)形成的。本研究通过分批吸附与Pb-L III一起研究了水铁矿-腐植酸(Fh-HA)复合材料中铅(Pb)的吸附行为EXAFS。我们报道通过共沉淀形成的复合材料与通过将HA吸附到预制Fh上形成的同等复合材料相比,具有更高的碳含量,更小的比表面积和更快的Pb吸附速率。与纯Fh相比,pH <5时,复合材料上的Pb吸附增强,并且共沉淀和吸附的复合材料吸附几乎等量的Pb。我们确定了在水铁矿表面上具有Pb-Fe键长为3.33Å的双齿边共享Pb络合物和在复合材料的HA组分上的双齿内球Pb络合物。随着pH值从4增加到6.5,更多的Pb离子与复合材料的HA组分相关。与共沉淀的复合材料相比,被吸附的有机部分螯合了更多的Pb离子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号