当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Novel Orally Available Asthma Drug Candidate That Reduces Smooth Muscle Constriction and Inflammation by Targeting GABAA Receptors in the Lung
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b01013 Gloria S Forkuo 1 , Amanda N Nieman 1 , Revathi Kodali 1 , Nicolas M Zahn 1 , Guanguan Li 1 , M S Rashid Roni 1 , Michael Rajesh Stephen 1 , Ted W Harris 1 , Rajwana Jahan 1 , Margaret L Guthrie 1 , Olivia B Yu 1 , Janet L Fisher 2 , Gene T Yocum 3 , Charles W Emala 3 , Douglas A Steeber 4 , Douglas C Stafford 1 , James M Cook 1 , Leggy A Arnold 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b01013 Gloria S Forkuo 1 , Amanda N Nieman 1 , Revathi Kodali 1 , Nicolas M Zahn 1 , Guanguan Li 1 , M S Rashid Roni 1 , Michael Rajesh Stephen 1 , Ted W Harris 1 , Rajwana Jahan 1 , Margaret L Guthrie 1 , Olivia B Yu 1 , Janet L Fisher 2 , Gene T Yocum 3 , Charles W Emala 3 , Douglas A Steeber 4 , Douglas C Stafford 1 , James M Cook 1 , Leggy A Arnold 1
Affiliation
|
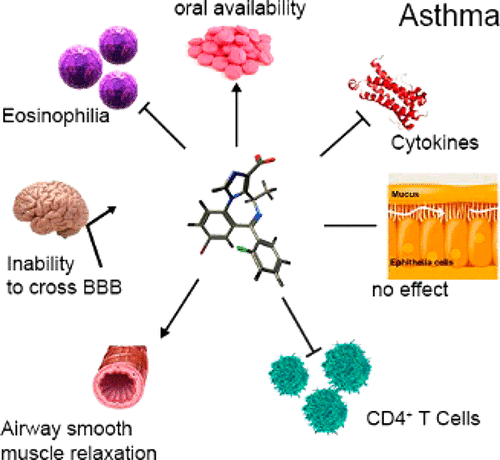
We describe lead compound MIDD0301 for the oral treatment of asthma based on previously developed positive allosteric α5β3γ2 selective GABAA receptor (GABAAR) ligands. MIDD0301 relaxed airway smooth muscle at single micromolar concentrations as demonstrated with ex vivo guinea pig tracheal rings. MIDD0301 also attenuated airway hyperresponsiveness (AHR) in an ovalbumin murine model of asthma by oral administration. Reduced numbers of eosinophils and macrophages were observed in mouse bronchoalveolar lavage fluid without changing mucous metaplasia. Importantly, lung cytokine expression of IL-17A, IL-4, and TNF-α were reduced for MIDD0301-treated mice without changing antiinflammatory cytokine IL-10 levels. Automated patch clamp confirmed amplification of GABA induced current mediated by α1–3,5β3γ2 GABAARs in the presence of MIDD0301. Pharmacodynamically, transmembrane currents of ex vivo CD4+ T cells from asthmatic mice were potentiated by MIDD0301 in the presence of GABA. The number of CD4+ T cells observed in the lung of MIDD0301-treated mice were reduced by an oral treatment of 20 mg/kg b.i.d. for 5 days. A half-life of almost 14 h was demonstrated by pharmacokinetic studies (PK) with no adverse CNS effects when treated mice were subjected to sensorimotor studies using the rotarod. PK studies also confirmed very low brain distribution. In conclusion, MIDD0301 represents a safe and improved oral asthma drug candidate that relaxes airway smooth muscle and attenuates inflammation in the lung leading to a reduction of AHR at a dosage lower than earlier reported GABAAR ligands.
中文翻译:

一种可通过靶向肺部 GABAA 受体减少平滑肌收缩和炎症的新型口服哮喘候选药物
我们描述了基于先前开发的正变构 α 5 β 3 γ 2选择性 GABA A受体 (GABA A ) 用于口服治疗哮喘的先导化合物 MIDD0301R)配体。MIDD0301 在单微摩尔浓度下松弛气道平滑肌,如离体豚鼠气管环所示。MIDD0301 还通过口服给药减轻卵清蛋白小鼠哮喘模型的气道高反应性 (AHR)。在没有改变粘液化生的小鼠支气管肺泡灌洗液中观察到嗜酸性粒细胞和巨噬细胞数量减少。重要的是,MIDD0301 处理的小鼠的肺细胞因子 IL-17A、IL-4 和 TNF-α 表达降低,而抗炎细胞因子 IL-10 水平没有改变。自动膜片钳证实由 α 1–3,5 β 3 γ 2 GABA A介导的 GABA 感应电流放大存在 MIDD0301 时的 Rs。在药效学上,来自哮喘小鼠的离体 CD4 + T 细胞的跨膜电流在 GABA 存在的情况下被 MIDD0301 增强。在 MIDD0301 处理小鼠的肺中观察到的CD4 + T 细胞数量通过口服 20 mg/kg bid 治疗 5 天而减少。药代动力学研究 (PK) 证明半衰期接近 14 小时,当使用旋转棒对治疗小鼠进行感觉运动研究时,没有不良 CNS 影响。PK 研究也证实了非常低的脑分布。总之,MIDD0301 代表了一种安全且改进的口服哮喘候选药物,可松弛气道平滑肌并减轻肺部炎症,导致 AHR 降低,剂量低于早先报道的 GABA AR配体。
更新日期:2018-03-26
中文翻译:

一种可通过靶向肺部 GABAA 受体减少平滑肌收缩和炎症的新型口服哮喘候选药物
我们描述了基于先前开发的正变构 α 5 β 3 γ 2选择性 GABA A受体 (GABA A ) 用于口服治疗哮喘的先导化合物 MIDD0301R)配体。MIDD0301 在单微摩尔浓度下松弛气道平滑肌,如离体豚鼠气管环所示。MIDD0301 还通过口服给药减轻卵清蛋白小鼠哮喘模型的气道高反应性 (AHR)。在没有改变粘液化生的小鼠支气管肺泡灌洗液中观察到嗜酸性粒细胞和巨噬细胞数量减少。重要的是,MIDD0301 处理的小鼠的肺细胞因子 IL-17A、IL-4 和 TNF-α 表达降低,而抗炎细胞因子 IL-10 水平没有改变。自动膜片钳证实由 α 1–3,5 β 3 γ 2 GABA A介导的 GABA 感应电流放大存在 MIDD0301 时的 Rs。在药效学上,来自哮喘小鼠的离体 CD4 + T 细胞的跨膜电流在 GABA 存在的情况下被 MIDD0301 增强。在 MIDD0301 处理小鼠的肺中观察到的CD4 + T 细胞数量通过口服 20 mg/kg bid 治疗 5 天而减少。药代动力学研究 (PK) 证明半衰期接近 14 小时,当使用旋转棒对治疗小鼠进行感觉运动研究时,没有不良 CNS 影响。PK 研究也证实了非常低的脑分布。总之,MIDD0301 代表了一种安全且改进的口服哮喘候选药物,可松弛气道平滑肌并减轻肺部炎症,导致 AHR 降低,剂量低于早先报道的 GABA AR配体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号