当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Through tissue imaging of a live breast cancer tumour model using handheld surface enhanced spatially offset resonance Raman spectroscopy (SESORRS)†
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1039/c8sc00994e Fay Nicolson 1, 2, 3, 4, 5 , Lauren E. Jamieson 1, 2, 3, 4, 5 , Samuel Mabbott 1, 2, 3, 4, 5 , Konstantinos Plakas 6, 7, 8, 9, 10 , Neil C. Shand 5, 11, 12 , Michael R. Detty 6, 7, 8, 9, 10 , Duncan Graham 1, 2, 3, 4, 5 , Karen Faulds 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1039/c8sc00994e Fay Nicolson 1, 2, 3, 4, 5 , Lauren E. Jamieson 1, 2, 3, 4, 5 , Samuel Mabbott 1, 2, 3, 4, 5 , Konstantinos Plakas 6, 7, 8, 9, 10 , Neil C. Shand 5, 11, 12 , Michael R. Detty 6, 7, 8, 9, 10 , Duncan Graham 1, 2, 3, 4, 5 , Karen Faulds 1, 2, 3, 4, 5
Affiliation
|
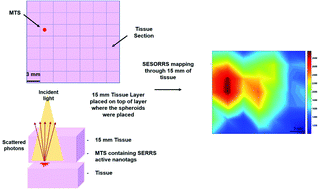
In order to improve patient survival and reduce the amount of unnecessary and traumatic biopsies, non-invasive detection of cancerous tumours is of imperative and urgent need. Multicellular tumour spheroids (MTS) can be used as an ex vivo cancer tumour model, to model in vivo nanoparticle (NP) uptake by the enhanced permeability and retention (EPR) effect. Surface enhanced spatially offset Raman spectroscopy (SESORS) combines both surface enhanced Raman spectroscopy (SERS) and spatially offset Raman spectroscopy (SORS) to yield enhanced Raman signals at much greater sub-surface levels. By utilizing a reporter that has an electronic transition in resonance with the laser frequency, surface enhanced resonance Raman scattering (SERRS) yields even greater enhancement in Raman signal. Using a handheld SORS spectrometer with back scattering optics, we demonstrate the detection of live breast cancer 3D MTS containing SERRS active NPs through 15 mm of porcine tissue. False color 2D heat intensity maps were used to determine tumour model location. In addition, we demonstrate the tracking of SERRS-active NPs through porcine tissue to depths of up to 25 mm. This unprecedented performance is due to the use of red-shifted chalcogenpyrylium-based Raman reporters to demonstrate the novel technique of surface enhanced spatially offset resonance Raman spectroscopy (SESORRS) for the first time. Our results demonstrate a significant step forward in the ability to detect vibrational fingerprints from a tumour model at depth through tissue. Such an approach offers significant promise for the translation of NPs into clinical applications for non-invasive disease diagnostics based on this new chemical principle of measurement.
中文翻译:

通过使用手持式表面增强的空间偏移共振拉曼光谱仪(SESORRS)对活的乳腺癌肿瘤模型进行组织成像†
为了提高患者的存活率并减少不必要的和创伤性的活组织检查,迫切需要紧急地无创检测癌性肿瘤。多细胞肿瘤球体(MTS)可以用作离体癌症肿瘤模型,以在体内建模增强的渗透性和保留(EPR)效果吸收纳米颗粒(NP)。表面增强的空间偏移拉曼光谱仪(SESORS)结合了表面增强的拉曼光谱仪(SERS)和空间偏移的拉曼光谱仪(SORS),可在更大的次表面水平上产生增强的拉曼信号。通过利用具有与激光频率共振的电子跃迁的报告分子,表面增强的共振拉曼散射(SERRS)可以产生更大的拉曼信号增强。使用带有背面散射光学元件的手持式SORS光谱仪,我们演示了通过15 mm的猪组织检测包含SERRS活性NP的活乳腺癌3D MTS。伪彩色2D热强度图用于确定肿瘤模型的位置。此外,我们证明了通过猪组织对SERRS活性NP的跟踪达到了25 mm的深度。这种空前的性能是由于使用了基于红移硫属吡啶鎓的拉曼报道分子来首次证明表面增强的空间偏移共振拉曼光谱学(SESORRS)的新技术。我们的结果表明,从组织深处的肿瘤模型中检测振动指纹的能力迈出了重要的一步。这种方法为基于这种新的化学测量原理将NP转化为用于非侵入性疾病诊断的临床应用提供了巨大的希望。这种空前的性能是由于使用了基于红移硫属吡啶鎓的拉曼报道分子来首次证明表面增强的空间偏移共振拉曼光谱学(SESORRS)的新技术。我们的研究结果表明,从深处穿过组织的肿瘤模型检测振动指纹的能力迈出了重要的一步。这种方法为基于这种新的化学测量原理将NP转化为用于非侵入性疾病诊断的临床应用提供了巨大的希望。这种空前的性能是由于使用了基于红移硫属吡啶鎓的拉曼报道分子来首次证明表面增强的空间偏移共振拉曼光谱学(SESORRS)的新技术。我们的研究结果表明,从深处穿过组织的肿瘤模型检测振动指纹的能力迈出了重要的一步。这种方法为基于这种新的化学测量原理将NP转化为用于非侵入性疾病诊断的临床应用提供了巨大的希望。
更新日期:2018-03-26
中文翻译:

通过使用手持式表面增强的空间偏移共振拉曼光谱仪(SESORRS)对活的乳腺癌肿瘤模型进行组织成像†
为了提高患者的存活率并减少不必要的和创伤性的活组织检查,迫切需要紧急地无创检测癌性肿瘤。多细胞肿瘤球体(MTS)可以用作离体癌症肿瘤模型,以在体内建模增强的渗透性和保留(EPR)效果吸收纳米颗粒(NP)。表面增强的空间偏移拉曼光谱仪(SESORS)结合了表面增强的拉曼光谱仪(SERS)和空间偏移的拉曼光谱仪(SORS),可在更大的次表面水平上产生增强的拉曼信号。通过利用具有与激光频率共振的电子跃迁的报告分子,表面增强的共振拉曼散射(SERRS)可以产生更大的拉曼信号增强。使用带有背面散射光学元件的手持式SORS光谱仪,我们演示了通过15 mm的猪组织检测包含SERRS活性NP的活乳腺癌3D MTS。伪彩色2D热强度图用于确定肿瘤模型的位置。此外,我们证明了通过猪组织对SERRS活性NP的跟踪达到了25 mm的深度。这种空前的性能是由于使用了基于红移硫属吡啶鎓的拉曼报道分子来首次证明表面增强的空间偏移共振拉曼光谱学(SESORRS)的新技术。我们的结果表明,从组织深处的肿瘤模型中检测振动指纹的能力迈出了重要的一步。这种方法为基于这种新的化学测量原理将NP转化为用于非侵入性疾病诊断的临床应用提供了巨大的希望。这种空前的性能是由于使用了基于红移硫属吡啶鎓的拉曼报道分子来首次证明表面增强的空间偏移共振拉曼光谱学(SESORRS)的新技术。我们的研究结果表明,从深处穿过组织的肿瘤模型检测振动指纹的能力迈出了重要的一步。这种方法为基于这种新的化学测量原理将NP转化为用于非侵入性疾病诊断的临床应用提供了巨大的希望。这种空前的性能是由于使用了基于红移硫属吡啶鎓的拉曼报道分子来首次证明表面增强的空间偏移共振拉曼光谱学(SESORRS)的新技术。我们的研究结果表明,从深处穿过组织的肿瘤模型检测振动指纹的能力迈出了重要的一步。这种方法为基于这种新的化学测量原理将NP转化为用于非侵入性疾病诊断的临床应用提供了巨大的希望。


























 京公网安备 11010802027423号
京公网安备 11010802027423号