当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transition-metal-free decarboxylative bromination of aromatic carboxylic acids†
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1039/c8sc01016a Jacob M. Quibell 1, 2, 3, 4 , Gregory J. P. Perry 1, 2, 3, 4, 5 , Diego M. Cannas 1, 2, 3, 4 , Igor Larrosa 1, 2, 3, 4
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1039/c8sc01016a Jacob M. Quibell 1, 2, 3, 4 , Gregory J. P. Perry 1, 2, 3, 4, 5 , Diego M. Cannas 1, 2, 3, 4 , Igor Larrosa 1, 2, 3, 4
Affiliation
|
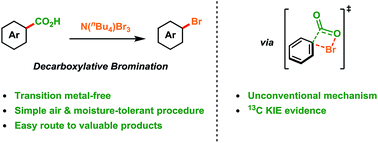
Methods for the conversion of aliphatic acids to alkyl halides have progressed significantly over the past century, however, the analogous decarboxylative bromination of aromatic acids has remained a longstanding challenge. The development of efficient methods for the synthesis of aryl bromides is of great importance as they are versatile reagents in synthesis and are present in many functional molecules. Herein we report a transition metal-free decarboxylative bromination of aromatic acids. The reaction is applicable to many electron-rich aromatic and heteroaromatic acids which have previously proved poor substrates for Hunsdiecker-type reactions. In addition, our preliminary mechanistic study suggests that radical intermediates are not involved in this reaction, which is in contrast to classical Hunsdiecker-type reactivity. Overall, the process demonstrates a useful method for producing valuable reagents from inexpensive and abundant starting materials.
中文翻译:

芳香族羧酸的无过渡金属脱羧溴化反应†
在过去的一个世纪中,将脂肪酸转化为卤代烷的方法取得了显着进展,然而,芳香族酸的类似脱羧溴化仍然是一个长期的挑战。开发有效的芳基溴化物合成方法非常重要,因为它们是合成中的通用试剂,并且存在于许多功能分子中。在此,我们报告了芳香族酸的无过渡金属脱羧溴化反应。该反应适用于许多富含电子的芳族和杂芳族酸,这些酸先前已被证明是Hunsdiecker型反应的不良底物。此外,我们的初步机理研究表明,自由基中间体不参与该反应,这与经典的Hunsdiecker型反应性相反。全面的,
更新日期:2018-03-26
中文翻译:

芳香族羧酸的无过渡金属脱羧溴化反应†
在过去的一个世纪中,将脂肪酸转化为卤代烷的方法取得了显着进展,然而,芳香族酸的类似脱羧溴化仍然是一个长期的挑战。开发有效的芳基溴化物合成方法非常重要,因为它们是合成中的通用试剂,并且存在于许多功能分子中。在此,我们报告了芳香族酸的无过渡金属脱羧溴化反应。该反应适用于许多富含电子的芳族和杂芳族酸,这些酸先前已被证明是Hunsdiecker型反应的不良底物。此外,我们的初步机理研究表明,自由基中间体不参与该反应,这与经典的Hunsdiecker型反应性相反。全面的,


























 京公网安备 11010802027423号
京公网安备 11010802027423号