当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct oxidative C–H alkynylation of N-carbamoyl tetrahydroisoquinolines and dihydroisoquinolines†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-03-24 00:00:00 , DOI: 10.1039/c8ob00373d Lei Chen 1, 2, 3, 4, 5 , Chuanxi Sun 1, 2, 3, 4 , Guidong Feng 1, 2, 3, 4 , Min Cao 4, 5, 6, 7, 8 , Shu-lei Zhao 4, 8, 9, 10 , Jun Yan 1, 2, 3, 4 , Ren-zhong Wan 1, 2, 3, 4 , Lei Liu 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-03-24 00:00:00 , DOI: 10.1039/c8ob00373d Lei Chen 1, 2, 3, 4, 5 , Chuanxi Sun 1, 2, 3, 4 , Guidong Feng 1, 2, 3, 4 , Min Cao 4, 5, 6, 7, 8 , Shu-lei Zhao 4, 8, 9, 10 , Jun Yan 1, 2, 3, 4 , Ren-zhong Wan 1, 2, 3, 4 , Lei Liu 4, 5, 6, 7
Affiliation
|
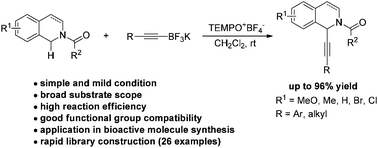
An efficient oxidative C–H alkynylation of N-carbamoyl tetrahydroisoquinolines mediated by a TEMPO oxoammonium salt has been established. A variety of electronically varied N-carbamoyl tetrahydroisoquinolines reacted with a range of alkynyl potassium trifluoroborates smoothly under mild metal-free conditions. Dihydroisoquinolines were also suitable components for the reaction. The synthetic applicability of the method for facile access to structurally diverse bioactive molecules was further demonstrated.
中文翻译:

N-氨基甲酰基四氢异喹啉和二氢异喹啉的 直接氧化C–H炔基化†
已经建立了由TEMPO氧铵盐介导的N-氨基甲酰基四氢异喹啉的有效氧化C-H炔基化反应。各种电子化的N-氨基甲酰基四氢异喹啉在温和的无金属条件下与一系列炔基三氟硼酸钾平滑反应。二氢异喹啉也是该反应的合适组分。进一步证明了该方法用于容易地获得结构上多样化的生物活性分子的合成适用性。
更新日期:2018-03-24
中文翻译:

N-氨基甲酰基四氢异喹啉和二氢异喹啉的 直接氧化C–H炔基化†
已经建立了由TEMPO氧铵盐介导的N-氨基甲酰基四氢异喹啉的有效氧化C-H炔基化反应。各种电子化的N-氨基甲酰基四氢异喹啉在温和的无金属条件下与一系列炔基三氟硼酸钾平滑反应。二氢异喹啉也是该反应的合适组分。进一步证明了该方法用于容易地获得结构上多样化的生物活性分子的合成适用性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号