当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the dioxygen activation and catalytic mechanism of the nickel-containing quercetinase†
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1039/c8cy00187a Hong Li 1, 2, 3, 4 , Xiya Wang 1, 2, 3, 4 , Ge Tian 1, 2, 3, 4 , Yongjun Liu 1, 2, 3, 4
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1039/c8cy00187a Hong Li 1, 2, 3, 4 , Xiya Wang 1, 2, 3, 4 , Ge Tian 1, 2, 3, 4 , Yongjun Liu 1, 2, 3, 4
Affiliation
|
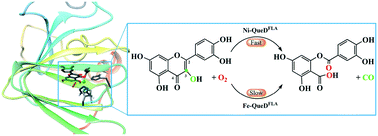
Quercetin 2,4-dioxygenase from Streptomyces sp. strain FLA (QueDFLA) is an enzyme of the monocupin family, which catalyzes the oxidative ring-cleaving reaction of quercetin using a nickel ion as the active site cofactor, and the iron ion that is necessary for most dioxygenases only shows low reactivity. To understand the reaction mechanism and the activation of dioxygen by the nickel ion, we performed QM/MM calculations to elucidate the reaction details and the special activation mechanism of this unique enzyme. Our calculations reveal two binding modes of dioxygen to the nickel ion, which can convert each other. Due to the overlap between the vacant d orbitals of nickel and the lone pair p orbitals of dioxygen and quercetin, electron transfer occurs from quercetin to dioxygen via the nickel center, thus, both dioxygen and quercetin can be activated by their binding to the nickel ion. On the basis of our calculations, the triplet reactant complex favors the catalytic reaction, and the whole reaction contains four elementary steps. In particular, a nonchemical process, the Op–Od bond rotation along the nickel center, is suggested to be rate-limiting with a free energy barrier of 19.9 kcal mol−1. NBO analysis reveals that it is the change of the coordination of Op with the nickel ion that leads to the high energy barrier of this process. In general, owing to the activation of the substrate and dioxygen by the nickel ion, the formation and collapse of the five-membered ring intermediate are quite easy, and the cleavage of O–O is in concert with the breaking of two C–C bonds. Furthermore, when the metal cofactor is replaced by an iron ion, the rate-limiting step switches from the Op–Od bond rotation to the collapse of the five-membered ring intermediate, corresponding to a free energy barrier of 30.3 kcal mol−1. This study sheds insight into the reaction mechanism of QueD and contributes to our general understanding of other nickel-containing enzymes.
中文翻译:

洞察含镍槲皮素酶的双氧激活和催化机制†
来自链霉菌属的槲皮素2,4-二加氧酶。菌株FLA(QueD FLA)是monocupin家族的一种酶,它使用镍离子作为活性位点辅因子催化槲皮素的氧化环裂解反应,而大多数双加氧酶所需的铁离子仅显示出低反应性。为了了解镍离子的反应机理和对双氧的活化作用,我们进行了QM / MM计算,以阐明反应细节以及这种独特酶的特殊活化机理。我们的计算揭示了双氧与镍离子的两种结合方式,它们可以相互转化。由于镍的空位d轨道与双氧和槲皮素的孤对p轨道之间存在重叠,因此电子从槲皮素转移到双氧因此,双氧和槲皮素都可以通过镍中心与镍离子结合而被活化。根据我们的计算,三重态反应物络合物有利于催化反应,整个反应包含四个基本步骤。特别是,非化学过程,即沿着镍中心的Op–Od键旋转,建议以19.9 kcal mol -1的自由能垒来限制速率。。NBO分析表明,正是Op与镍离子配位的变化导致了该过程的高能垒。通常,由于镍离子对底物和双氧的活化,五元环中间体的形成和崩解非常容易,O-O的裂解与两个C-C的断裂相一致。债券。此外,当金属辅因子被铁离子替代时,限速步骤从Op-Od键旋转切换为五元环中间体的坍塌,这对应于30.3 kcal mol -1的自由能垒。这项研究深入了解了QueD的反应机理,有助于我们对其他含镍酶的一般理解。
更新日期:2018-03-23
中文翻译:

洞察含镍槲皮素酶的双氧激活和催化机制†
来自链霉菌属的槲皮素2,4-二加氧酶。菌株FLA(QueD FLA)是monocupin家族的一种酶,它使用镍离子作为活性位点辅因子催化槲皮素的氧化环裂解反应,而大多数双加氧酶所需的铁离子仅显示出低反应性。为了了解镍离子的反应机理和对双氧的活化作用,我们进行了QM / MM计算,以阐明反应细节以及这种独特酶的特殊活化机理。我们的计算揭示了双氧与镍离子的两种结合方式,它们可以相互转化。由于镍的空位d轨道与双氧和槲皮素的孤对p轨道之间存在重叠,因此电子从槲皮素转移到双氧因此,双氧和槲皮素都可以通过镍中心与镍离子结合而被活化。根据我们的计算,三重态反应物络合物有利于催化反应,整个反应包含四个基本步骤。特别是,非化学过程,即沿着镍中心的Op–Od键旋转,建议以19.9 kcal mol -1的自由能垒来限制速率。。NBO分析表明,正是Op与镍离子配位的变化导致了该过程的高能垒。通常,由于镍离子对底物和双氧的活化,五元环中间体的形成和崩解非常容易,O-O的裂解与两个C-C的断裂相一致。债券。此外,当金属辅因子被铁离子替代时,限速步骤从Op-Od键旋转切换为五元环中间体的坍塌,这对应于30.3 kcal mol -1的自由能垒。这项研究深入了解了QueD的反应机理,有助于我们对其他含镍酶的一般理解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号