当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Toward a Ferrous Iron-Cleavable Linker for Antibody–Drug Conjugates
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00242 Benjamin Spangler 1 , Toni Kline 2 , Jeffrey Hanson 2 , Xiaofan Li 2 , Sihong Zhou 2 , James A. Wells , Aaron K. Sato 2 , Adam R. Renslo
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00242 Benjamin Spangler 1 , Toni Kline 2 , Jeffrey Hanson 2 , Xiaofan Li 2 , Sihong Zhou 2 , James A. Wells , Aaron K. Sato 2 , Adam R. Renslo
Affiliation
|
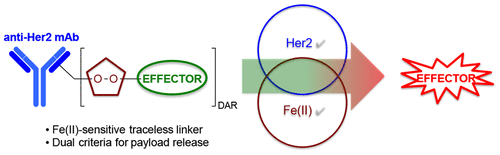
Antibody–drug conjugates (ADCs) are antigen-targeted therapeutics that employ antibodies to deliver potent, cytotoxic effectors to cells with potentially high specificity. While promising clinical results have been achieved, significant pitfalls remain including internalization of ADCs in nontargeted cells expressing target antigen, which can limit therapeutic windows. Novel ADC linkers that are cleaved selectively in cancer cells but not in normal cells could minimize collateral damage caused by ADC uptake in nontargeted tissues. Here, we describe a prototypical ADC linker based on an Fe(II)-reactive 1,2,4-trioxolane scaffold (TRX) that by itself has demonstrated tumor-selective activity in preclinical cancer models. We prepared TRX-linked ADCs by site-selective conjugation to two sites in trastuzumab and compared their activity in Her2 positive and negative cells to ADC controls based on established linker chemistry. Our results confirm that the TRX moiety efficiently releases its payload following ADC uptake, affording picomolar potencies in antigen-positive cells. We also identified a destabilizing interaction between these initial TRX linkers and nearby antibody residues and suggest an approach to improve upon these prototypical designs.
中文翻译:

走向铁-可裂解的抗体-药物结合连接物
抗体-药物偶联物(ADC)是一种以抗原为靶标的治疗药物,采用抗体将潜在的高细胞毒性效应子以潜在的高特异性传递给细胞。尽管已经取得了令人鼓舞的临床结果,但仍然存在重大陷阱,包括ADC在表达靶抗原的非靶细胞中的内在化,这可能会限制治疗窗口。在癌细胞中而非正常细胞中选择性切割的新型ADC连接子可以最大程度地减少非目标组织中ADC摄取引起的附带损害。在这里,我们描述了一个基于Fe(II)反应性1,2,4-三氧戊环骨架(TRX)的原型ADC接头,该接头本身已在临床前癌症模型中证明了肿瘤选择性活性。我们通过与曲妥珠单抗中的两个位点选择性缀合来制备TRX链接的ADC,并根据已建立的接头化学将它们在Her2阳性和阴性细胞中的活性与ADC对照进行了比较。我们的结果证实,TRX部分在ADC摄取后有效释放了其有效负载,从而在抗原阳性细胞中提供了皮摩尔的效力。我们还确定了这些初始TRX接头和附近的抗体残基之间的不稳定相互作用,并提出了一种改进这些原型设计的方法。
更新日期:2018-03-23
中文翻译:

走向铁-可裂解的抗体-药物结合连接物
抗体-药物偶联物(ADC)是一种以抗原为靶标的治疗药物,采用抗体将潜在的高细胞毒性效应子以潜在的高特异性传递给细胞。尽管已经取得了令人鼓舞的临床结果,但仍然存在重大陷阱,包括ADC在表达靶抗原的非靶细胞中的内在化,这可能会限制治疗窗口。在癌细胞中而非正常细胞中选择性切割的新型ADC连接子可以最大程度地减少非目标组织中ADC摄取引起的附带损害。在这里,我们描述了一个基于Fe(II)反应性1,2,4-三氧戊环骨架(TRX)的原型ADC接头,该接头本身已在临床前癌症模型中证明了肿瘤选择性活性。我们通过与曲妥珠单抗中的两个位点选择性缀合来制备TRX链接的ADC,并根据已建立的接头化学将它们在Her2阳性和阴性细胞中的活性与ADC对照进行了比较。我们的结果证实,TRX部分在ADC摄取后有效释放了其有效负载,从而在抗原阳性细胞中提供了皮摩尔的效力。我们还确定了这些初始TRX接头和附近的抗体残基之间的不稳定相互作用,并提出了一种改进这些原型设计的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号