当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bioassay- and Chemistry-Guided Isolation of Scalemic Caged Prenylxanthones from the Leaves of Garcinia bracteata
Journal of Natural Products ( IF 5.1 ) Pub Date : 2018-03-22 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00454 Sheng-Li Niu 1 , Da-Hong Li , Xin-Yu Li , Yue-Tong Wang , Sheng-Ge Li , Jiao Bai , Yue-Hu Pei , Yong-Kui Jing , Zhan-Lin Li , Hui-Ming Hua
Journal of Natural Products ( IF 5.1 ) Pub Date : 2018-03-22 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00454 Sheng-Li Niu 1 , Da-Hong Li , Xin-Yu Li , Yue-Tong Wang , Sheng-Ge Li , Jiao Bai , Yue-Hu Pei , Yong-Kui Jing , Zhan-Lin Li , Hui-Ming Hua
Affiliation
|
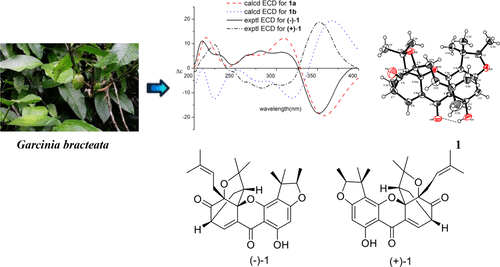
With bioassay- and chemistry-guided fractionation, seven new caged prenylxanthones including two scalemic mixtures, epiisobractatin (1), 13-hydroxyisobractatin (2), 13-hydroxyepiisobractatin (3), 8-methoxy-8,8a-dihydrobractatin (4), 8-ethoxy-8,8a-dihydrobractatin (5), garcibracteatone (6), and 8-methoxy-8,8a-dihydroneobractiatin (7), and the eight known compounds 8–15 were isolated from the leaves of Garcinia bracteata. The structures were unambiguously elucidated through analysis of spectroscopic data. The 2D structures and relative configurations of 1 and 5 were confirmed by X-ray crystallographic analysis. The separation of the enantiomers of 1–5 was accomplished by chiral-phase HPLC. The absolute configurations of the enantiomers of 1 and 5 were assigned by comparison of the experimental and calculated electronic circular dichroism (ECD) spectra. The absolute configurations of the related compounds were determined via comparisons of their ECD data with those of the enantiomers of 1 and 5, respectively. Notably, compound 7, with a neo caged skeleton, is the first representative of a novel type of caged xanthone lacking a Δ8(8a) double bond. The isolated compounds exhibited significant cell growth inhibitory activities in vitro against human leukemic HL-60 and K562 cell lines, with GI50 values ranging from 0.2 to 8.8 μM. A preliminary structure–activity relationship is discussed.
中文翻译:

生物测定和化学指导从藤黄叶片中分离出鳞状笼状苯并氧杂蒽酮。
与bioassay-和化学引导分级,七个新笼prenylxanthones包括二消旋混合物,epiisobractatin(1),13-hydroxyisobractatin(2),13-hydroxyepiisobractatin(3),8-甲氧基8,8a-dihydrobractatin(4), 8-乙氧基8,8a-dihydrobractatin(5),garcibracteatone(6),以及8-甲氧基8,8a-dihydroneobractiatin(7),和八个已知化合物8 - 15从叶分离的藤黄苞。通过光谱数据分析清楚地阐明了结构。二维结构和相对构型1通过X射线晶体学分析确认5和5。的对映体的分离1 - 5通过手性相HPLC来完成。通过比较实验值和计算的电子圆二色性(ECD)光谱,确定1和5对映体的绝对构型。相关化合物的绝对构型是通过将它们的ECD数据分别与1和5的对映体进行比较来确定的。值得注意的是,具有新笼状骨架的化合物7是缺乏Δ8 (8a)的新型笼状黄酮的首个代表。双键。分离的化合物在体外对人白血病HL-60和K562细胞系表现出显着的细胞生长抑制活性,GI 50值为0.2至8.8μM。讨论了初步的构效关系。
更新日期:2018-03-22
中文翻译:

生物测定和化学指导从藤黄叶片中分离出鳞状笼状苯并氧杂蒽酮。
与bioassay-和化学引导分级,七个新笼prenylxanthones包括二消旋混合物,epiisobractatin(1),13-hydroxyisobractatin(2),13-hydroxyepiisobractatin(3),8-甲氧基8,8a-dihydrobractatin(4), 8-乙氧基8,8a-dihydrobractatin(5),garcibracteatone(6),以及8-甲氧基8,8a-dihydroneobractiatin(7),和八个已知化合物8 - 15从叶分离的藤黄苞。通过光谱数据分析清楚地阐明了结构。二维结构和相对构型1通过X射线晶体学分析确认5和5。的对映体的分离1 - 5通过手性相HPLC来完成。通过比较实验值和计算的电子圆二色性(ECD)光谱,确定1和5对映体的绝对构型。相关化合物的绝对构型是通过将它们的ECD数据分别与1和5的对映体进行比较来确定的。值得注意的是,具有新笼状骨架的化合物7是缺乏Δ8 (8a)的新型笼状黄酮的首个代表。双键。分离的化合物在体外对人白血病HL-60和K562细胞系表现出显着的细胞生长抑制活性,GI 50值为0.2至8.8μM。讨论了初步的构效关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号