Biomaterials ( IF 14.0 ) Pub Date : 2018-03-21 , DOI: 10.1016/j.biomaterials.2018.03.035 Jiajia Tan , Zhengyu Deng , Guhuan Liu , Jinming Hu , Shiyong Liu
|
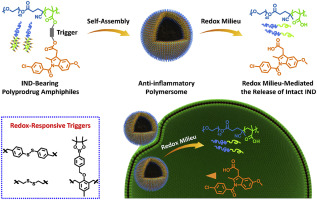
Inflammation serves as a natural defense mechanism to protect living organisms from infectious diseases. Nonsteroidal anti-inflammatory drugs (NSAIDs) can help relieve inflammatory reactions and are clinically used to treat pain, fever, and inflammation, whereas long-term use of NSAIDs may lead to severe side effects including gastrointestinal damage and cardiovascular toxicity. Therefore, it is of increasing importance to configure new dosing strategies and alleviate the side effects of NSAIDs. Towards this goal, glutathione (GSH)-responsive disulfide bonds and hydrogen peroxide (H2O2)-reactive phenylboronic ester linkages were utilized as triggering moieties in this work to design redox-responsive prodrug monomers and polyprodrug amphiphiles based on indomethacin (IND) drug. Note that IND is a widely prescribed NSAID in the clinic. Starting from three types of redox-reactive IND prodrug monomers, redox-responsive polyprodrug amphiphiles were synthesized through reversible addition-fragmentation chain transfer (RAFT) polymerizations of prodrug monomers using poly(ethylene oxide) (PEO)-based macroRAFT agent. The resultant polyprodrug amphiphiles with high IND loading contents (>33 wt%) could self-assemble into polymersomes with PEO shielding coronas and redox-responsive bilayer membranes composed of IND prodrugs. Upon incubation with GSH or H2O2, controlled release of intact IND in the active form from polyprodrug polymersomes was actuated by GSH-mediated disulfide cleavage reaction and H2O2-mediated oxidation of phenylboronic ester moieties, respectively, followed by self-immolative degradation events. Furthermore, in vitro studies at the cellular level revealed that redox-responsive polymersomes could efficiently relieve inflammatory responses induced by lipopolysaccharide (LPS) in RAW264.7 macrophage cells.
中文翻译:

具有炎症触发消炎痛释放特性的氧化还原反应性多药两亲物的消炎聚合物小体
炎症是保护生物体免受传染病侵害的自然防御机制。非甾体类抗炎药(NSAIDs)可帮助缓解炎症反应,临床上可用于治疗疼痛,发烧和炎症,而长期使用NSAIDs可能导致严重的副作用,包括胃肠道损害和心血管毒性。因此,配置新的给药策略并减轻NSAID的副作用变得越来越重要。为了实现这一目标,谷胱甘肽(GSH)响应的二硫键和过氧化氢(H 2 O 2反应性苯基硼酸酯键被用作触发部分,以基于吲哚美辛(IND)的药物设计氧化还原响应前药单体和多药两亲物。请注意,IND是临床上广泛使用的NSAID。从三种类型的氧化还原反应性IND前药单体开始,使用基于聚环氧乙烷(PEO)的macroRAFT剂,通过前药单体的可逆加成-断裂链转移(RAFT)聚合反应合成氧化还原响应性多药前两亲物。所得的具有高IND负载量(> 33 wt%)的多药前两亲物可以自组装成具有PEO屏蔽电晕和由IND前药组成的氧化还原反应性双层膜的聚合物囊泡。与GSH或H 2 O 2孵育后分别通过GSH介导的二硫键裂解反应和H 2 O 2介导的苯硼酸酯部分的氧化,激活了活性形式的完整IND从聚前药聚合物小体中的受控释放,然后发生了自焚降解事件。此外,在细胞水平的体外研究表明,氧化还原反应性聚合物囊泡可以有效缓解RAW264.7巨噬细胞中脂多糖(LPS)诱导的炎症反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号