当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molybdenum Burial Mechanism in Sulfidic Sediments: Iron-Sulfide Pathway
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00016 Trent P. Vorlicek 1 , George R. Helz 2 , Anthony Chappaz 3 , Pakou Vue 1 , Austin Vezina 1 , Wayland Hunter 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00016 Trent P. Vorlicek 1 , George R. Helz 2 , Anthony Chappaz 3 , Pakou Vue 1 , Austin Vezina 1 , Wayland Hunter 1
Affiliation
|
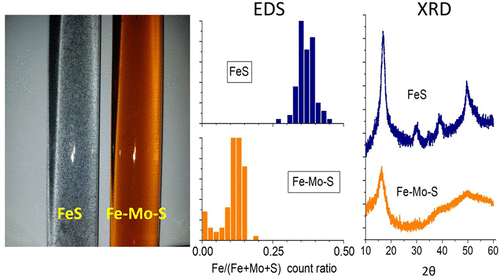
Relative to continental crust, sediments underlying sulfidic marine waters are molybdenum-rich, a property preserved in the rock record and useful for characterizing paleoenvironments. The enrichment mechanism is not agreed upon but is attributed at least partly to deposition of Fe–Mo–S compounds, which are as yet uncharacterized. Here, we determine the composition and stability of colloidal Fe–Mo–S precipitates formed at mildly basic pH and H2S(aq) > 10–5 M. The first product consists simply of FeMoS4, with Ksp = 10–14.95. Within hours, FeMoS4 irreversibly transforms by internal self-reduction to a Mo(IV) product of similar composition. The reduced product is insoluble in 1 M HCl but soluble in concentrated HNO3, implying that it would be recovered with pyrite in a common assay of sediments. X-ray absorption fine structure data show that Mo(IV) in the colloids is coordinated by a split first shell of about five sulfur atoms at average distances of 2.31 and 2.46 Å and in its second shell by an iron atom at about 2.80 Å. These properties resemble those determined for Mo in modern anoxic lake sediments and in Phanerozoic black shales. The atomic environment around Mo suggests that the colloidal products may be inorganic polymers containing cuboid, Fe2Mo2S44+ cores. Such materials are so far unreported by mineralogists, although a rare mineral, jordisite, may be a related, but more Mo-rich material. The low solubility of FeMoS4 makes it a feasible precipitate in euxinic waters like those in the modern Black Sea. We propose that colloids similar to those studied here could account for Mo-enrichment in euxinic basin sediments and black shales.
中文翻译:

硫化物沉积物中的钼埋藏机理:硫化铁途径
相对于大陆壳,硫化海水下的沉积物富含钼,该属性保留在岩石记录中,可用于表征古环境。富集机理尚未达成共识,但至少部分归因于尚未表征的Fe-Mo-S化合物的沉积。在这里,我们确定在适度碱性pH值和H 2 S(aq)> 10 –5 M时形成的胶体Fe–Mo–S沉淀物的组成和稳定性。第一种产品仅由FeMoS 4组成,K sp = 10 –14.95。。数小时内,FeMoS 4通过内部自我还原不可逆地转化为组成相似的Mo(IV)产物。还原后的产物不溶于1 M HCl,但溶于浓HNO 3,这意味着在常规的沉积物中可将其与黄铁矿一起回收。X射线吸收精细结构数据表明,胶体中的Mo(IV)与大约5个硫原子的裂开的第一壳配位,平均距离为2.31和2.46Å,第二个壳中的Mo(IV)与铁原子配成约2.80Å。这些性质类似于现代缺氧湖泊沉积物和生代黑色页岩中的钼。Mo周围的原子环境表明,胶体产物可能是含有长方体,Fe 2 Mo 2 S 4 4+的无机聚合物。核心。迄今为止,矿物学家尚未报道过这种材料,尽管稀有的矿物(芒硝)可能是一种相关的,但富含钼的材料。FeMoS 4的低溶解度使其成为像现代黑海那样在富氧水域中可行的沉淀物。我们认为,与此处研究的胶体相似,胶体可以解释富营养化盆地沉积物和黑色页岩中的钼富集。
更新日期:2018-03-20
中文翻译:

硫化物沉积物中的钼埋藏机理:硫化铁途径
相对于大陆壳,硫化海水下的沉积物富含钼,该属性保留在岩石记录中,可用于表征古环境。富集机理尚未达成共识,但至少部分归因于尚未表征的Fe-Mo-S化合物的沉积。在这里,我们确定在适度碱性pH值和H 2 S(aq)> 10 –5 M时形成的胶体Fe–Mo–S沉淀物的组成和稳定性。第一种产品仅由FeMoS 4组成,K sp = 10 –14.95。。数小时内,FeMoS 4通过内部自我还原不可逆地转化为组成相似的Mo(IV)产物。还原后的产物不溶于1 M HCl,但溶于浓HNO 3,这意味着在常规的沉积物中可将其与黄铁矿一起回收。X射线吸收精细结构数据表明,胶体中的Mo(IV)与大约5个硫原子的裂开的第一壳配位,平均距离为2.31和2.46Å,第二个壳中的Mo(IV)与铁原子配成约2.80Å。这些性质类似于现代缺氧湖泊沉积物和生代黑色页岩中的钼。Mo周围的原子环境表明,胶体产物可能是含有长方体,Fe 2 Mo 2 S 4 4+的无机聚合物。核心。迄今为止,矿物学家尚未报道过这种材料,尽管稀有的矿物(芒硝)可能是一种相关的,但富含钼的材料。FeMoS 4的低溶解度使其成为像现代黑海那样在富氧水域中可行的沉淀物。我们认为,与此处研究的胶体相似,胶体可以解释富营养化盆地沉积物和黑色页岩中的钼富集。



























 京公网安备 11010802027423号
京公网安备 11010802027423号