当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Incorporation of Hydrogen Bond Angle Dependency into the Generalized Solvation Free Energy Density Model
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1021/acs.jcim.7b00410 Songling Ma 1, 2 , Sungbo Hwang 1 , Sehan Lee 1, 3 , William E. Acree 4 , Kyoung Tai No 1, 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1021/acs.jcim.7b00410 Songling Ma 1, 2 , Sungbo Hwang 1 , Sehan Lee 1, 3 , William E. Acree 4 , Kyoung Tai No 1, 2
Affiliation
|
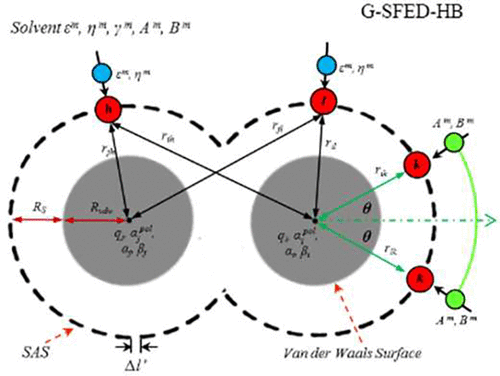
To describe the physically realistic solvation free energy surface of a molecule in a solvent, a generalized version of the solvation free energy density (G-SFED) calculation method has been developed. In the G-SFED model, the contribution from the hydrogen bond (HB) between a solute and a solvent to the solvation free energy was calculated as the product of the acidity of the donor and the basicity of the acceptor of an HB pair. The acidity and basicity parameters of a solute were derived using the summation of acidities and basicities of the respective acidic and basic functional groups of the solute, and that of the solvent was experimentally determined. Although the contribution of HBs to the solvation free energy could be evenly distributed to grid points on the surface of a molecule, the G-SFED model was still inadequate to describe the angle dependency of the HB of a solute with a polarizable continuum solvent. To overcome this shortcoming of the G-SFED model, the contribution of HBs was formulated using the geometric parameters of the grid points described in the HB coordinate system of the solute. We propose an HB angle dependency incorporated into the G-SFED model, i.e., the G-SFED-HB model, where the angular-dependent acidity and basicity densities are defined and parametrized with experimental data. The G-SFED-HB model was then applied to calculate the solvation free energies of organic molecules in water, various alcohols and ethers, and the log P values of diverse organic molecules, including peptides and a protein. Both the G-SFED model and the G-SFED-HB model reproduced the experimental solvation free energies with similar accuracy, whereas the distributions of the SFED on the molecular surface calculated by the G-SFED and G-SFED-HB models were quite different, especially for molecules having HB donors or acceptors. Since the angle dependency of HBs was included in the G-SFED-HB model, the SFED distribution of the G-SFED-HB model is well described as compared to that of the G-SFED model.
中文翻译:

将氢键角依赖性纳入广义溶剂化自由能密度模型
为了描述分子在溶剂中的物理现实的溶剂化自由能表面,已开发了溶剂化自由能密度(G-SFED)计算方法的通用版本。在G-SFED模型中,溶质和溶剂之间的氢键(HB)对溶剂化自由能的贡献计算为供体酸度与HB对受体碱性的乘积。溶质的酸度和碱度参数是使用溶质各自的酸性和碱性官能团的酸度和碱度之和得出的,并通过实验确定了溶剂的酸度和碱度参数。尽管HBs对溶剂化自由能的贡献可以均匀地分布在分子表面的网格点上,G-SFED模型仍然不足以描述溶质与可极化连续介质溶剂的HB的角度依赖性。为了克服G-SFED模型的这一缺点,使用溶质的HB坐标系中描述的网格点的几何参数来确定HBs的贡献。我们提出将HB角度依赖性纳入G-SFED模型(即G-SFED-HB模型)中,其中定义了角度依赖性酸度和碱度密度,并通过实验数据进行了参数化。然后应用G-SFED-HB模型计算水,各种醇和醚中有机分子的溶剂化自由能,以及log HBs的贡献是使用溶质的HB坐标系中描述的网格点的几何参数来确定的。我们提出将HB角度依赖性纳入G-SFED模型(即G-SFED-HB模型)中,其中定义了角度依赖性酸度和碱度密度,并通过实验数据进行了参数化。然后,使用G-SFED-HB模型来计算水,各种醇和醚中有机分子的溶剂化自由能,以及log HBs的贡献是使用溶质的HB坐标系中描述的网格点的几何参数来确定的。我们提出将HB角度依赖性纳入G-SFED模型(即G-SFED-HB模型)中,其中定义了角度依赖性酸度和碱度密度,并通过实验数据进行了参数化。然后,使用G-SFED-HB模型来计算水,各种醇和醚中有机分子的溶剂化自由能,以及log包括肽和蛋白质在内的各种有机分子的P值。G-SFED模型和G-SFED-HB模型均以相似的精度再现了实验溶剂化自由能,而G-SFED和G-SFED-HB模型计算出的SFED在分子表面的分布却大不相同。 ,特别是对于具有HB供体或受体的分子。由于HBs的角度依赖性已包含在G-SFED-HB模型中,因此与G-SFED模型相比,可以很好地描述G-SFED-HB模型的SFED分布。
更新日期:2018-03-21
中文翻译:

将氢键角依赖性纳入广义溶剂化自由能密度模型
为了描述分子在溶剂中的物理现实的溶剂化自由能表面,已开发了溶剂化自由能密度(G-SFED)计算方法的通用版本。在G-SFED模型中,溶质和溶剂之间的氢键(HB)对溶剂化自由能的贡献计算为供体酸度与HB对受体碱性的乘积。溶质的酸度和碱度参数是使用溶质各自的酸性和碱性官能团的酸度和碱度之和得出的,并通过实验确定了溶剂的酸度和碱度参数。尽管HBs对溶剂化自由能的贡献可以均匀地分布在分子表面的网格点上,G-SFED模型仍然不足以描述溶质与可极化连续介质溶剂的HB的角度依赖性。为了克服G-SFED模型的这一缺点,使用溶质的HB坐标系中描述的网格点的几何参数来确定HBs的贡献。我们提出将HB角度依赖性纳入G-SFED模型(即G-SFED-HB模型)中,其中定义了角度依赖性酸度和碱度密度,并通过实验数据进行了参数化。然后应用G-SFED-HB模型计算水,各种醇和醚中有机分子的溶剂化自由能,以及log HBs的贡献是使用溶质的HB坐标系中描述的网格点的几何参数来确定的。我们提出将HB角度依赖性纳入G-SFED模型(即G-SFED-HB模型)中,其中定义了角度依赖性酸度和碱度密度,并通过实验数据进行了参数化。然后,使用G-SFED-HB模型来计算水,各种醇和醚中有机分子的溶剂化自由能,以及log HBs的贡献是使用溶质的HB坐标系中描述的网格点的几何参数来确定的。我们提出将HB角度依赖性纳入G-SFED模型(即G-SFED-HB模型)中,其中定义了角度依赖性酸度和碱度密度,并通过实验数据进行了参数化。然后,使用G-SFED-HB模型来计算水,各种醇和醚中有机分子的溶剂化自由能,以及log包括肽和蛋白质在内的各种有机分子的P值。G-SFED模型和G-SFED-HB模型均以相似的精度再现了实验溶剂化自由能,而G-SFED和G-SFED-HB模型计算出的SFED在分子表面的分布却大不相同。 ,特别是对于具有HB供体或受体的分子。由于HBs的角度依赖性已包含在G-SFED-HB模型中,因此与G-SFED模型相比,可以很好地描述G-SFED-HB模型的SFED分布。


























 京公网安备 11010802027423号
京公网安备 11010802027423号