Chemosphere ( IF 8.8 ) Pub Date : 2018-03-20 , DOI: 10.1016/j.chemosphere.2018.03.119 Zhenyu Shi , Jing Zhang , Liang Zhu
|
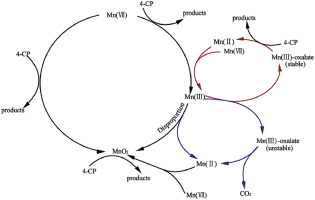
The role of oxalate in 4-chlorophenol (4-CP) oxidation by permanganate (Mn(VII)) was explored in this study. The performance of oxalate was heavily depended on pH and oxalate concentration. 4-CP degradation by Mn(VII) was significantly enhanced at pH 4.0–6.0 in the presence of oxalate, while negligible influence was observed at pH 7.0–9.0. The oxalate plays a dual role in Mn(VII) oxidation over the pH range of 4.0–6.0: one is the chelate, which coordinates with Mn(III) to form Mn(III)-oxalate complexes, and the other is the reductant, which reacts with Mn(III) to form Mn(II). The stable Mn(III)-oxalate complexes can work as an efficient oxidant for 4-CP. While their unstable counterparts, due to the lower concentration of oxalate or the higher pH, would auto-decomposed to MnO2 and Mn(II), and then the MnO2 works as both a catalyst and an oxidant for the decomposition of 4-CP.
中文翻译:

草酸盐在4-氯苯酚高锰酸盐氧化中的作用
本研究探讨了草酸盐在高氯酸盐(Mn(VII))氧化4-氯苯酚(4-CP)中的作用。草酸盐的性能在很大程度上取决于pH值和草酸盐浓度。在草酸存在下,pH 4.0-6.0时,Mn(VII)对4-CP的降解作用明显增强,而在pH 7.0-9.0时,观察到的影响可忽略不计。草酸盐在4.0-6.0的pH范围内对Mn(VII)的氧化起双重作用:一种是螯合物,与Mn(III)配合形成Mn(III)-草酸盐络合物,另一种是还原剂,它与Mn(III)反应形成Mn(II)。稳定的草酸锰(III)配合物可作为4-CP的有效氧化剂。虽然它们不稳定的对应物,由于较低的草酸盐浓度或较高的pH值,它们会自动分解为MnO 2和Mn(II),然后分解为MnO。2作为4-CP分解的催化剂和氧化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号