当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single-Molecule Detection Reveals Different Roles of Skp and SurA as Chaperones
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acschembio.8b00097 Geng Li 1, 2 , Chenhui He 2, 3 , Peixuan Bu 1, 2 , Huimin Bi 1, 2 , Sichen Pan 1, 2 , Ronghua Sun 1, 2 , Xin Sheng Zhao 1, 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acschembio.8b00097 Geng Li 1, 2 , Chenhui He 2, 3 , Peixuan Bu 1, 2 , Huimin Bi 1, 2 , Sichen Pan 1, 2 , Ronghua Sun 1, 2 , Xin Sheng Zhao 1, 2
Affiliation
|
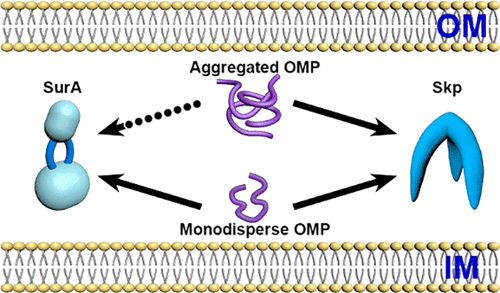
Skp and SurA are both periplasmic chaperones involved in the biogenesis of Escherichia coli β-barrel outer membrane proteins (OMPs). It is commonly assumed that SurA plays a major role whereas Skp is a minor factor. However, there is no molecular evidence for whether their roles are redundant. Here, by using different dilution methods, we obtained monodisperse and aggregated forms of OmpC and studied their interactions with Skp and SurA by single-molecule fluorescence resonance energy transfer and fluorescence correlation spectroscopy. We found that Skp can dissolve aggregated OmpC while SurA cannot convert aggregated OmpC into the monodisperse form and the conformations of OmpC recognized by the two chaperones as well as their stoichiometries of binding are different. Our study demonstrates the functional distinctions between Skp and SurA. In particular, the role of Skp is not redundant and is probably more significant under stress conditions.
中文翻译:

单分子检测揭示了Skp和SurA作为伴侣的不同作用。
Skp和SurA都是与大肠杆菌生物发生有关的周质伴侣β桶外膜蛋白(OMP)。通常认为SurA起主要作用,而Skp是次要因素。但是,没有分子证据证明它们的作用是否多余。在这里,通过使用不同的稀释方法,我们获得了OmpC的单分散和聚集形式,并通过单分子荧光共振能量转移和荧光相关光谱研究了它们与Skp和SurA的相互作用。我们发现Skp可以溶解聚集的OmpC,而SurA不能将聚集的OmpC转化为单分散形式,并且两个分子伴侣识别的OmpC构象及其结合的化学计量是不同的。我们的研究证明了Skp和SurA之间的功能区别。尤其是,
更新日期:2018-03-15
中文翻译:

单分子检测揭示了Skp和SurA作为伴侣的不同作用。
Skp和SurA都是与大肠杆菌生物发生有关的周质伴侣β桶外膜蛋白(OMP)。通常认为SurA起主要作用,而Skp是次要因素。但是,没有分子证据证明它们的作用是否多余。在这里,通过使用不同的稀释方法,我们获得了OmpC的单分散和聚集形式,并通过单分子荧光共振能量转移和荧光相关光谱研究了它们与Skp和SurA的相互作用。我们发现Skp可以溶解聚集的OmpC,而SurA不能将聚集的OmpC转化为单分散形式,并且两个分子伴侣识别的OmpC构象及其结合的化学计量是不同的。我们的研究证明了Skp和SurA之间的功能区别。尤其是,



























 京公网安备 11010802027423号
京公网安备 11010802027423号