当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
α-Substituted Benzylic Complexes of Palladium(II) as Precursors of Palladium Hydrides
Organometallics ( IF 2.8 ) Pub Date : 2018-03-19 , DOI: 10.1021/acs.organomet.8b00066 Blanca Martín-Ruiz 1 , Ignacio Pérez-Ortega 1 , Ana C. Albéniz 1
Organometallics ( IF 2.8 ) Pub Date : 2018-03-19 , DOI: 10.1021/acs.organomet.8b00066 Blanca Martín-Ruiz 1 , Ignacio Pérez-Ortega 1 , Ana C. Albéniz 1
Affiliation
|
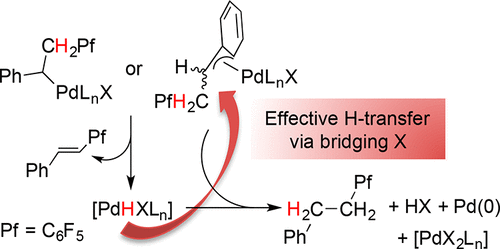
The adoption of a pseudoallylic (η3) form makes palladium benzylic derivatives a class of stabilized palladium alkyls that can undergo β-H elimination reactions in a more controlled way. α-(Pentafluorophenylmethyl)benzyl palladium complexes have been studied, and they decompose by β-H elimination to give palladium hydrides that, depending on the auxiliary ligands, can: (a) transmetalate to another palladium atom and, by reductive elimination, give hydrogenated products; this process is favored for a combination of bridging ligands (i.e., halogens) and low coordinating ligands. (b) Be used as a hydride source and get trapped by a diene to give palladium allylic derivatives. The presence of carbon monoxide does not induce a β-H elimination reaction, and only CO insertion into the Pd–benzyl bond to give acyl derivatives is observed.
中文翻译:

钯(II)的α-取代的苯甲酸络合物作为钯氢化物的前体
通过一个pseudoallylic的(η 3)形式使钯苄基衍生物成为一类稳定的钯烷基,可以以更可控的方式进行β-H消除反应。已经研究了α-(五氟苯基甲基)苄基钯配合物,它们通过β-H消除反应分解生成氢化钯,取决于辅助配体,氢化钯可以:(a)过渡金属化为另一个钯原子,并通过还原消除进行氢化产品; 对于桥接配体(即卤素)和低配位配体的组合,此方法是有利的。(b)用作氢化物源,并被二烯捕获,得到钯烯丙基衍生物。一氧化碳的存在不会引起β-H消除反应,仅观察到CO插入Pd-苄基键以生成酰基衍生物。
更新日期:2018-06-03
中文翻译:

钯(II)的α-取代的苯甲酸络合物作为钯氢化物的前体
通过一个pseudoallylic的(η 3)形式使钯苄基衍生物成为一类稳定的钯烷基,可以以更可控的方式进行β-H消除反应。已经研究了α-(五氟苯基甲基)苄基钯配合物,它们通过β-H消除反应分解生成氢化钯,取决于辅助配体,氢化钯可以:(a)过渡金属化为另一个钯原子,并通过还原消除进行氢化产品; 对于桥接配体(即卤素)和低配位配体的组合,此方法是有利的。(b)用作氢化物源,并被二烯捕获,得到钯烯丙基衍生物。一氧化碳的存在不会引起β-H消除反应,仅观察到CO插入Pd-苄基键以生成酰基衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号