当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Glutathione-induced amino-activatable micellar photosensitization platform for synergistic redox modulation and photodynamic therapy†
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1039/c8bm00094h Ruiwei Guo 1, 2, 3, 4, 5 , Guang Yang 1, 2, 3, 4, 5 , Zujian Feng 1, 2, 3, 4, 5 , Yujie Zhu 1, 2, 3, 4, 5 , Pengxiang Yang 6, 7, 8, 9, 10 , Huijuan Song 6, 7, 8, 9, 10 , Weiwei Wang 6, 7, 8, 9, 10 , Pingsheng Huang 6, 7, 8, 9, 10 , Jianhua Zhang 1, 2, 3, 4, 5
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1039/c8bm00094h Ruiwei Guo 1, 2, 3, 4, 5 , Guang Yang 1, 2, 3, 4, 5 , Zujian Feng 1, 2, 3, 4, 5 , Yujie Zhu 1, 2, 3, 4, 5 , Pengxiang Yang 6, 7, 8, 9, 10 , Huijuan Song 6, 7, 8, 9, 10 , Weiwei Wang 6, 7, 8, 9, 10 , Pingsheng Huang 6, 7, 8, 9, 10 , Jianhua Zhang 1, 2, 3, 4, 5
Affiliation
|
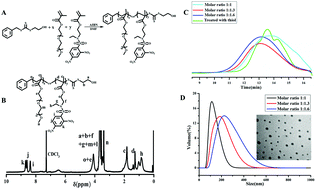
In recent years, photodynamic therapy (PDT) was considered to be a promising cancer treatment modality, however, the therapeutic efficiency was often attenuated by the intrinsic antioxidant defense systems. Herein, a kind of novel glutathione-induced amino-activatable micelle was designed, which was expected to weaken the antioxidant capacity and in the meantime release the photosensitizer by the exhaustion of intracellular glutathione (GSH). The amphiphilic poly(ethylene glycol)-(2-((2,4-dinitro-N-(ethyl) phenyl)sulfonamido) ethyl methacrylate) copolymers were synthesized and assembled into a core–shell nano structure in aqueous media. The nano structure demonstrated high sensitivity and selectivity to bio-thiols in vitro and in vivo. Subsequently, pheophorbide a (PhA) was encapsulated as the model photosensitizer. Upon internalization by HepG2 cells, the strongly electron-withdrawing 2,4-dinitrobenzenesulfonyl groups on the PADEE segments were readily cleaved by GSH, during which time the secondary amino groups (pKb = 11.32) were recovered and completely protonated, leading to disassembly of the micelles and rapid release of PhA. Importantly, the consumption of GSH weakened the intracellular antioxidant capacity, resulting in the synergetic accumulation of reactive oxygen species (ROS) under laser irradiation. As a result, this micellar photosensitization system could overcome the antioxidant capacity of advanced stage tumors through a simultaneous extrinsic and intrinsic strategy, facilitating therapeutic efficiency. These results demonstrate that the as-designed micelles provide a versatile photosensitization platform for on-demand PDT.
中文翻译:

谷胱甘肽诱导的氨基活化胶束光敏化平台,用于协同氧化还原调节和光动力疗法†
近年来,光动力疗法(PDT)被认为是一种有前途的癌症治疗方法,但是,固有的抗氧化剂防御系统常常削弱了治疗效率。本文设计了一种新型的谷胱甘肽诱导的氨基可活化胶束,有望减弱其抗氧化能力,同时通过细胞内谷胱甘肽(GSH)的耗尽而释放出光敏剂。合成了两亲性聚乙二醇-(2-((2,4-二硝基-N-(乙基)苯基)磺酰胺基)甲基丙烯酸乙酯)共聚物,并在水性介质中组装成核-壳纳米结构。纳米结构在体外和体内显示出对生物硫醇的高敏感性和选择性。随后,将脱镁叶绿酸a(PhA)封装为模型光敏剂。在被HepG2细胞内化后,PADEE片段上强吸电子的2,4-二硝基苯磺酰基很容易被GSH裂解,在此期间,仲氨基(p K b= 11.32)被回收并完全质子化,从而导致胶束的分解和PhA的快速释放。重要的是,GSH的消耗削弱了细胞内的抗氧化能力,导致激光照射下活性氧(ROS)的协同积累。结果,该胶束光敏系统可以通过同时进行外在和内在策略来克服晚期肿瘤的抗氧化能力,从而提高治疗效率。这些结果表明,设计好的胶束为按需PDT提供了通用的光敏平台。
更新日期:2018-03-20
中文翻译:

谷胱甘肽诱导的氨基活化胶束光敏化平台,用于协同氧化还原调节和光动力疗法†
近年来,光动力疗法(PDT)被认为是一种有前途的癌症治疗方法,但是,固有的抗氧化剂防御系统常常削弱了治疗效率。本文设计了一种新型的谷胱甘肽诱导的氨基可活化胶束,有望减弱其抗氧化能力,同时通过细胞内谷胱甘肽(GSH)的耗尽而释放出光敏剂。合成了两亲性聚乙二醇-(2-((2,4-二硝基-N-(乙基)苯基)磺酰胺基)甲基丙烯酸乙酯)共聚物,并在水性介质中组装成核-壳纳米结构。纳米结构在体外和体内显示出对生物硫醇的高敏感性和选择性。随后,将脱镁叶绿酸a(PhA)封装为模型光敏剂。在被HepG2细胞内化后,PADEE片段上强吸电子的2,4-二硝基苯磺酰基很容易被GSH裂解,在此期间,仲氨基(p K b= 11.32)被回收并完全质子化,从而导致胶束的分解和PhA的快速释放。重要的是,GSH的消耗削弱了细胞内的抗氧化能力,导致激光照射下活性氧(ROS)的协同积累。结果,该胶束光敏系统可以通过同时进行外在和内在策略来克服晚期肿瘤的抗氧化能力,从而提高治疗效率。这些结果表明,设计好的胶束为按需PDT提供了通用的光敏平台。


























 京公网安备 11010802027423号
京公网安备 11010802027423号