Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2018-03-19 , DOI: 10.1016/j.bmcl.2018.03.047 Vijeta Sharma , Nagarjuna Amarnath , Swapnil Shukla , R. Ayana , Naveen Kumar , Nisha Yadav , Deepika Kannan , Seema Sehrawat , Soumya Pati , Bimlesh Lochab , Shailja Singh
|
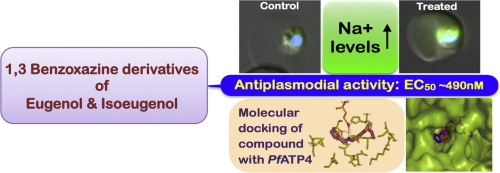
Development of new class of anti-malarial drugs is an essential requirement for the elimination of malaria. Bioactive components present in medicinal plants and their chemically modified derivatives could be a way forward towards the discovery of effective anti-malarial drugs. Herein, we describe a new class of compounds, 1,3-benzoxazine derivatives of pharmacologically active phytophenols eugenol (compound 3) and isoeugenol (compound 4) synthesised on the principles of green chemistry, as anti-malarials. Compound 4, showed highest anti-malarial activity with no cytotoxicity towards mammalian cells. Compound 4 induced alterations in the intracellular Na+ levels and mitochondrial depolarisation in intraerythrocytic Plasmodium falciparum leading to cell death. Knowing P-type cation ATPase PfATP4 is a regulator for sodium homeostasis, binding of compound 3, compound 4 and eugenol to PfATP4 was analysed by molecular docking studies. Compounds showed binding to the catalytic pocket of PfATP4, however compound 4 showed stronger binding due to the presence of propylene functionality, which corroborates its higher anti-malarial activity. Furthermore, anti-malarial half maximal effective concentration of compound 4 was reduced to 490 nM from 17.54 µM with nanomaterial graphene oxide. Altogether, this study presents anti-plasmodial potential of benzoxazine derivatives of phytophenols and establishes disruption of parasite sodium homeostasis as their mechanism of action.
中文翻译:

植物酚的苯并恶嗪衍生物通过钠稳态破坏显示抗血浆活性
开发新型抗疟疾药物是消除疟疾的基本要求。药用植物及其化学修饰衍生物中存在的生物活性成分可能是发现有效抗疟疾药物的一种方法。在此,我们将一类新化合物描述为具有抗疟疾作用的药理活性植物酚丁子香酚(化合物3)和异丁香酚(化合物4)的1,3-苯并恶嗪衍生物。化合物4显示出最高的抗疟活性,对哺乳动物细胞无细胞毒性。化合物4诱导细胞内Na +的改变红细胞内恶性疟原虫中的血红蛋白水平和线粒体去极化导致细胞死亡。已知P型阳离子ATP酶PfATP4是钠稳态的调节剂,通过分子对接研究分析了化合物3,化合物4和丁子香酚与PfATP4的结合。化合物显示与PfATP4的催化口袋结合,但是化合物4由于存在丙烯官能团而显示出更强的结合力,这证实了其较高的抗疟活性。此外,化合物4的抗疟疾半数最大有效浓度用纳米材料氧化石墨烯从17.54 µM降至490 nM。总的来说,这项研究提出了植物酚的苯并恶嗪衍生物的抗疟原虫的潜力,并建立了破坏寄生虫钠稳态的作用机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号