PLOS ONE ( IF 3.7 ) Pub Date : 2018-03-19 , DOI: 10.1371/journal.pone.0194620 Anneliese S. Ashhurst , Thaigarajan Parumasivam , John Gar Yan Chan , Leon C. W. Lin , Manuela Flórido , Nicholas P. West , Hak-Kim Chan , Warwick J. Britton
|
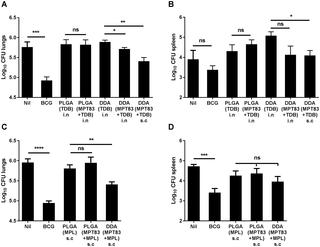
Tuberculosis places a staggering burden on human health globally. The new World Health Organisation End-TB Strategy has highlighted the urgent need for more effective TB vaccines to improve control of the disease. Protein-based subunit vaccines offer potential as safe and effective generators of protective immunity, and the use of particulate vaccine formulation and delivery by the pulmonary route may enhance local immunogenicity. In this study, novel particulate subunit vaccines were developed utilising biodegradable poly(lactic-co-glycolic acid) (PLGA) slow-release particles as carriers for the Mycobacterium tuberculosis lipoprotein MPT83, together with the adjuvants trehalose-dibehenate (TDB) or Monophosphoryl lipid A (MPL). Following delivery by the pulmonary or subcutaneous routes, the immunogenicity and protective efficacy of these vaccines were assessed in a murine model of M. tuberculosis infection. When delivered peripherally, these vaccines induced modest, antigen-specific Th1 and Th17 responses, but strong anti-MPT83 antibody responses. Mucosal delivery of the PLGA(MPT83) vaccine, with or without TDB, increased antigen-specific Th17 responses in the lungs, however, PLGA-encapsulated vaccines did not provide protection against M. tuberculosis challenge. By contrast, peripheral delivery of DDA liposomes containing MPT83 and TDB or MPL, stimulated both Th1 and Th17 responses and generated protection against M. tuberculosis challenge. Therefore, PLGA-formulated vaccines primarily stimulate strong humoral immunity, or Th17 responses if used mucosally, and may be a suitable carrier for vaccines against extracellular pathogens. This study emphasises the critical nature of the vaccine carrier, adjuvant and route of delivery for optimising vaccine efficacy against TB.
中文翻译:

PLGA微粒亚基结核疫苗可促进体液和Th17反应,但不能增强对结核分枝杆菌感染的控制
结核病给全球人类健康带来了巨大负担。新的世界卫生组织《结核病最终防治战略》强调了迫切需要更有效的结核病疫苗以改善对疾病的控制。基于蛋白质的亚单位疫苗提供了潜在的安全有效的保护性免疫产生剂,使用微粒疫苗制剂和通过肺部途径给药可增强局部免疫原性。在这项研究中,新颖的颗粒的亚单位疫苗被开发利用生物可降解的聚(乳酸-共-glycolic乙酸)(PLGA)缓释颗粒作为载体的结核分枝杆菌脂蛋白MPT83,以及佐剂海藻糖二山hen酸酯(TDB)或单磷酰脂质A(MPL)。通过肺或皮下途径递送后,在M1鼠模型中评估了这些疫苗的免疫原性和保护效力。结核感染。当以外围方式递送时,这些疫苗诱导适度的,抗原特异性的Th1和Th17反应,但强烈的抗MPT83抗体反应。在有或没有TDB的情况下,粘膜递送PLGA(MPT83)疫苗会增加肺中抗原特异性Th17反应,但是,PLGA封装的疫苗不能提供针对M的保护作用。结核挑战。相比之下,含有MPT83和TDB或MPL的DDA脂质体的外周递送刺激了Th1和Th17反应,并产生了针对M的保护作用。结核病挑战。因此,PLGA配制的疫苗主要刺激强烈的体液免疫或粘膜使用的Th17应答,并且可能是针对细胞外病原体的疫苗的合适载体。这项研究强调了疫苗载体,佐剂和递送途径对优化针对结核病的疫苗效力的关键性质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号