当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single-Molecule Fluorescence Detection of the Epidermal Growth Factor Receptor in Membrane Discs.
Biochemistry ( IF 2.9 ) Pub Date : 2018-04-06 , DOI: 10.1021/acs.biochem.8b00089 Steven D Quinn 1 , Shwetha Srinivasan 1 , Jesse B Gordon 1 , Wei He 2 , Kermit L Carraway 3 , Matthew A Coleman 2, 4 , Gabriela S Schlau-Cohen 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-04-06 , DOI: 10.1021/acs.biochem.8b00089 Steven D Quinn 1 , Shwetha Srinivasan 1 , Jesse B Gordon 1 , Wei He 2 , Kermit L Carraway 3 , Matthew A Coleman 2, 4 , Gabriela S Schlau-Cohen 1
Affiliation
|
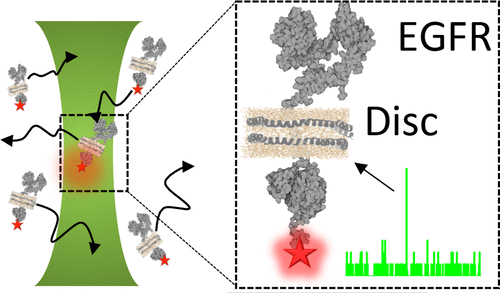
The epidermal growth factor receptor (EGFR) is critical to normal cellular signaling pathways. Moreover, it has been implicated in a range of pathologies, including cancer. As a result, it is the primary target of many anticancer drugs. One limitation to the design and development of these drugs has been the lack of molecular-level information about the interactions and conformational dynamics of EGFR. To overcome this limitation, this work reports the construction and characterization of functional, fluorescently labeled, and full-length EGFR in model membrane nanolipoprotein particles (NLPs) for in vitro fluorescence studies. To demonstrate the utility of the system, we investigate ATP-EGFR interactions. We observe that ATP binds at the catalytic site providing a means to measure a range of distances between the catalytic site and the C-terminus via Förster resonance energy transfer (FRET). These ATP-based experiments suggest a range of conformations of the C-terminus that may be a function of the phosphorylation state for EGFR. This work is a proof-of-principle demonstration of single-molecule studies as a noncrystallographic assay for EGFR interactions in real-time and under near-physiological conditions. The diverse nature of EGFR interactions means that new tools at the molecular level have the potential to significantly enhance our understanding of receptor pathology and are of utmost importance for cancer-related drug discovery.
中文翻译:

膜片中表皮生长因子受体的单分子荧光检测。
表皮生长因子受体(EGFR)对于正常的细胞信号通路至关重要。而且,它与多种病理学有关,包括癌症。结果,它是许多抗癌药物的主要靶标。这些药物设计和开发的限制之一是缺乏有关EGFR相互作用和构象动力学的分子水平信息。为了克服这一限制,这项工作报告了在模型膜纳米脂蛋白颗粒(NLP)中用于体外荧光研究的功能性,荧光标记和全长EGFR的构建和表征。为了证明该系统的实用性,我们研究了ATP-EGFR相互作用。我们观察到ATP在催化位点结合,从而提供了一种通过Förster共振能量转移(FRET)测量催化位点与C末端之间距离范围的手段。这些基于ATP的实验表明,C末端的一系列构象可能是EGFR磷酸化状态的函数。这项工作是单分子研究的原理证明,是作为实时和近乎生理条件下的EGFR相互作用的非晶体分析方法。EGFR相互作用的多样性意味着在分子水平上的新工具有可能显着增强我们对受体病理学的理解,并且对于与癌症相关的药物发现至关重要。这些基于ATP的实验表明,C末端的一系列构象可能是EGFR磷酸化状态的函数。这项工作是单分子研究的原理证明,是作为实时和近乎生理条件下的EGFR相互作用的非晶体分析方法。EGFR相互作用的多样性意味着在分子水平上的新工具有可能显着增强我们对受体病理学的理解,并且对于与癌症相关的药物发现至关重要。这些基于ATP的实验表明,C末端的一系列构象可能是EGFR磷酸化状态的函数。这项工作是单分子研究的原理证明,是作为实时和近乎生理条件下的EGFR相互作用的非晶体分析方法。EGFR相互作用的多样性意味着在分子水平上的新工具有可能显着增强我们对受体病理学的理解,并且对于与癌症相关的药物发现至关重要。
更新日期:2018-03-19
中文翻译:

膜片中表皮生长因子受体的单分子荧光检测。
表皮生长因子受体(EGFR)对于正常的细胞信号通路至关重要。而且,它与多种病理学有关,包括癌症。结果,它是许多抗癌药物的主要靶标。这些药物设计和开发的限制之一是缺乏有关EGFR相互作用和构象动力学的分子水平信息。为了克服这一限制,这项工作报告了在模型膜纳米脂蛋白颗粒(NLP)中用于体外荧光研究的功能性,荧光标记和全长EGFR的构建和表征。为了证明该系统的实用性,我们研究了ATP-EGFR相互作用。我们观察到ATP在催化位点结合,从而提供了一种通过Förster共振能量转移(FRET)测量催化位点与C末端之间距离范围的手段。这些基于ATP的实验表明,C末端的一系列构象可能是EGFR磷酸化状态的函数。这项工作是单分子研究的原理证明,是作为实时和近乎生理条件下的EGFR相互作用的非晶体分析方法。EGFR相互作用的多样性意味着在分子水平上的新工具有可能显着增强我们对受体病理学的理解,并且对于与癌症相关的药物发现至关重要。这些基于ATP的实验表明,C末端的一系列构象可能是EGFR磷酸化状态的函数。这项工作是单分子研究的原理证明,是作为实时和近乎生理条件下的EGFR相互作用的非晶体分析方法。EGFR相互作用的多样性意味着在分子水平上的新工具有可能显着增强我们对受体病理学的理解,并且对于与癌症相关的药物发现至关重要。这些基于ATP的实验表明,C末端的一系列构象可能是EGFR磷酸化状态的函数。这项工作是单分子研究的原理证明,是作为实时和近乎生理条件下的EGFR相互作用的非晶体分析方法。EGFR相互作用的多样性意味着在分子水平上的新工具有可能显着增强我们对受体病理学的理解,并且对于与癌症相关的药物发现至关重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号