当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
From 4-nitrotoluene and 4,4′-dinitrobibenzyl to E-4,4′-dinitrostilbene: an electrochemical approach
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8nj00131f Iluminada Gallardo 1, 2, 3, 4 , Ana Belén Gómez 1, 2, 3, 4 , Gonzalo Guirado 1, 2, 3, 4 , Adrián Lariño 1, 2, 3, 4 , Miquel Moreno 1, 2, 3, 4 , Manuel Ortigosa 1, 2, 3, 4 , Sergio Soler 1, 2, 3, 4
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8nj00131f Iluminada Gallardo 1, 2, 3, 4 , Ana Belén Gómez 1, 2, 3, 4 , Gonzalo Guirado 1, 2, 3, 4 , Adrián Lariño 1, 2, 3, 4 , Miquel Moreno 1, 2, 3, 4 , Manuel Ortigosa 1, 2, 3, 4 , Sergio Soler 1, 2, 3, 4
Affiliation
|
The dianions formed by the electroreduction of Z-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO2, E-O2NC6H4CH
CHC6H4NO2, E-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO2, O2NC6H4CH2–CH2C6H4NO2 or O2NC6H4CMeH–CMeHC6H4NO2, as well as the anion radical arising from 4-nitrotoluene, are stable, in the time scale of cyclic voltammetry (DMF + 0.10 M NBu4BF4). However, in the electrolysis time scale (from minutes to hours), only the dianion O2NC6H4CMeH–CMeHC6H4NO22− remains stable, since the reduced species, Z-O2NC6H4CH
CHC6H4NO2, O2NC6H4CH2–CH2C6H4NO2 or O2NC6H4CMeH–CMeHC6H4NO2, as well as the anion radical arising from 4-nitrotoluene, are stable, in the time scale of cyclic voltammetry (DMF + 0.10 M NBu4BF4). However, in the electrolysis time scale (from minutes to hours), only the dianion O2NC6H4CMeH–CMeHC6H4NO22− remains stable, since the reduced species, Z-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO22−, O2NC6H4CH2–CH2C6H4NO22− or O2NC6H4Me˙−, evolve to form the E-O2NC6H4CH
CHC6H4NO22−, O2NC6H4CH2–CH2C6H4NO22− or O2NC6H4Me˙−, evolve to form the E-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO22− dianion. This intermediate is recovered as the neutral species E-O2NC6H4CH
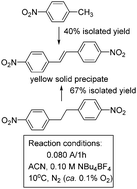
CHC6H4NO22− dianion. This intermediate is recovered as the neutral species E-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO2 with concomitant water reduction after the work-up with water, as demonstrated by combined electrolysis, cyclic voltammetry experiments, UV-spectroelectrochochemistry and theoretical calculations. Bulk electrolysis under optimized conditions (ACN + 0.10 M NBu4BF4) provides 40% and 67% isolated yields of E-4,4′-dinitrostilbene from 4-nitrotoluene and 4,4′-dinitrobibenzyl, respectively.
CHC6H4NO2 with concomitant water reduction after the work-up with water, as demonstrated by combined electrolysis, cyclic voltammetry experiments, UV-spectroelectrochochemistry and theoretical calculations. Bulk electrolysis under optimized conditions (ACN + 0.10 M NBu4BF4) provides 40% and 67% isolated yields of E-4,4′-dinitrostilbene from 4-nitrotoluene and 4,4′-dinitrobibenzyl, respectively.
中文翻译:

从4-硝基甲苯和4,4'-二硝基联苄到E -4,4'-二硝基苯乙烯:电化学方法
通过Z -O 2 NC 6 H 4 CH![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CHC 6 H 4 NO 2,E -O 2 NC 6 H 4 CH
CHC 6 H 4 NO 2,E -O 2 NC 6 H 4 CH ![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CHC 6 H 4 NO 2,O 2 NC 6 H 4 CH 2 -CH 2的电还原形成的阴离子。C 6 H 4 NO 2或O 2 NC 6 H 4 CMeH–CMeHC 6 H在循环伏安法(DMF + 0.10 M NBu 4 BF 4)的时间范围内, 4 NO 2以及由4-硝基甲苯产生的阴离子自由基是稳定的。但是,在电解时间范围内(从几分钟到几小时),只有二价阴离子O 2 NC 6 H 4 CMeH–CMeHC 6 H 4 NO 2 2-保持稳定,因为还原的物质Z -O 2 NC 6 H 4 CHCHC 6 H 4 NO 2 2-,O 2 NC 6 H
CHC 6 H 4 NO 2,O 2 NC 6 H 4 CH 2 -CH 2的电还原形成的阴离子。C 6 H 4 NO 2或O 2 NC 6 H 4 CMeH–CMeHC 6 H在循环伏安法(DMF + 0.10 M NBu 4 BF 4)的时间范围内, 4 NO 2以及由4-硝基甲苯产生的阴离子自由基是稳定的。但是,在电解时间范围内(从几分钟到几小时),只有二价阴离子O 2 NC 6 H 4 CMeH–CMeHC 6 H 4 NO 2 2-保持稳定,因为还原的物质Z -O 2 NC 6 H 4 CHCHC 6 H 4 NO 2 2-,O 2 NC 6 H![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 4 CH 2 -CH 2 C ^ 6 ħ 4 NO 2 2-或O的2 NC 6 H ^ 4 ME - ,进化以形成ë -O 2 NC 6 H ^ 4 CH
4 CH 2 -CH 2 C ^ 6 ħ 4 NO 2 2-或O的2 NC 6 H ^ 4 ME - ,进化以形成ë -O 2 NC 6 H ^ 4 CH![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CHC 6 H ^ 4 NO 2 2-的二价阴离子。该中间体被回收为中性物质E -O 2 NC 6 H 4 CH
CHC 6 H ^ 4 NO 2 2-的二价阴离子。该中间体被回收为中性物质E -O 2 NC 6 H 4 CH![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CHC 6 H 4 NO 2加水后伴随的水减少,如通过组合电解,循环伏安法实验,UV光谱电化学法和理论计算所证明的。在最佳条件下(ACN + 0.10 M NBu 4 BF 4)进行本体电解可分别从4-硝基甲苯和4,4'-二硝基联苄中分离出40%和67%的E -4,4'-二硝基sti。
CHC 6 H 4 NO 2加水后伴随的水减少,如通过组合电解,循环伏安法实验,UV光谱电化学法和理论计算所证明的。在最佳条件下(ACN + 0.10 M NBu 4 BF 4)进行本体电解可分别从4-硝基甲苯和4,4'-二硝基联苄中分离出40%和67%的E -4,4'-二硝基sti。
更新日期:2018-03-19
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO2, E-O2NC6H4CH
CHC6H4NO2, E-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO2, O2NC6H4CH2–CH2C6H4NO2 or O2NC6H4CMeH–CMeHC6H4NO2, as well as the anion radical arising from 4-nitrotoluene, are stable, in the time scale of cyclic voltammetry (DMF + 0.10 M NBu4BF4). However, in the electrolysis time scale (from minutes to hours), only the dianion O2NC6H4CMeH–CMeHC6H4NO22− remains stable, since the reduced species, Z-O2NC6H4CH
CHC6H4NO2, O2NC6H4CH2–CH2C6H4NO2 or O2NC6H4CMeH–CMeHC6H4NO2, as well as the anion radical arising from 4-nitrotoluene, are stable, in the time scale of cyclic voltammetry (DMF + 0.10 M NBu4BF4). However, in the electrolysis time scale (from minutes to hours), only the dianion O2NC6H4CMeH–CMeHC6H4NO22− remains stable, since the reduced species, Z-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO22−, O2NC6H4CH2–CH2C6H4NO22− or O2NC6H4Me˙−, evolve to form the E-O2NC6H4CH
CHC6H4NO22−, O2NC6H4CH2–CH2C6H4NO22− or O2NC6H4Me˙−, evolve to form the E-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO22− dianion. This intermediate is recovered as the neutral species E-O2NC6H4CH
CHC6H4NO22− dianion. This intermediate is recovered as the neutral species E-O2NC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC6H4NO2 with concomitant water reduction after the work-up with water, as demonstrated by combined electrolysis, cyclic voltammetry experiments, UV-spectroelectrochochemistry and theoretical calculations. Bulk electrolysis under optimized conditions (ACN + 0.10 M NBu4BF4) provides 40% and 67% isolated yields of E-4,4′-dinitrostilbene from 4-nitrotoluene and 4,4′-dinitrobibenzyl, respectively.
CHC6H4NO2 with concomitant water reduction after the work-up with water, as demonstrated by combined electrolysis, cyclic voltammetry experiments, UV-spectroelectrochochemistry and theoretical calculations. Bulk electrolysis under optimized conditions (ACN + 0.10 M NBu4BF4) provides 40% and 67% isolated yields of E-4,4′-dinitrostilbene from 4-nitrotoluene and 4,4′-dinitrobibenzyl, respectively.
中文翻译:

从4-硝基甲苯和4,4'-二硝基联苄到E -4,4'-二硝基苯乙烯:电化学方法
通过Z -O 2 NC 6 H 4 CH
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CHC 6 H 4 NO 2,E -O 2 NC 6 H 4 CH
CHC 6 H 4 NO 2,E -O 2 NC 6 H 4 CH ![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CHC 6 H 4 NO 2,O 2 NC 6 H 4 CH 2 -CH 2的电还原形成的阴离子。C 6 H 4 NO 2或O 2 NC 6 H 4 CMeH–CMeHC 6 H在循环伏安法(DMF + 0.10 M NBu 4 BF 4)的时间范围内, 4 NO 2以及由4-硝基甲苯产生的阴离子自由基是稳定的。但是,在电解时间范围内(从几分钟到几小时),只有二价阴离子O 2 NC 6 H 4 CMeH–CMeHC 6 H 4 NO 2 2-保持稳定,因为还原的物质Z -O 2 NC 6 H 4 CHCHC 6 H 4 NO 2 2-,O 2 NC 6 H
CHC 6 H 4 NO 2,O 2 NC 6 H 4 CH 2 -CH 2的电还原形成的阴离子。C 6 H 4 NO 2或O 2 NC 6 H 4 CMeH–CMeHC 6 H在循环伏安法(DMF + 0.10 M NBu 4 BF 4)的时间范围内, 4 NO 2以及由4-硝基甲苯产生的阴离子自由基是稳定的。但是,在电解时间范围内(从几分钟到几小时),只有二价阴离子O 2 NC 6 H 4 CMeH–CMeHC 6 H 4 NO 2 2-保持稳定,因为还原的物质Z -O 2 NC 6 H 4 CHCHC 6 H 4 NO 2 2-,O 2 NC 6 H![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) 4 CH 2 -CH 2 C ^ 6 ħ 4 NO 2 2-或O的2 NC 6 H ^ 4 ME - ,进化以形成ë -O 2 NC 6 H ^ 4 CH
4 CH 2 -CH 2 C ^ 6 ħ 4 NO 2 2-或O的2 NC 6 H ^ 4 ME - ,进化以形成ë -O 2 NC 6 H ^ 4 CH![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CHC 6 H ^ 4 NO 2 2-的二价阴离子。该中间体被回收为中性物质E -O 2 NC 6 H 4 CH
CHC 6 H ^ 4 NO 2 2-的二价阴离子。该中间体被回收为中性物质E -O 2 NC 6 H 4 CH![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) CHC 6 H 4 NO 2加水后伴随的水减少,如通过组合电解,循环伏安法实验,UV光谱电化学法和理论计算所证明的。在最佳条件下(ACN + 0.10 M NBu 4 BF 4)进行本体电解可分别从4-硝基甲苯和4,4'-二硝基联苄中分离出40%和67%的E -4,4'-二硝基sti。
CHC 6 H 4 NO 2加水后伴随的水减少,如通过组合电解,循环伏安法实验,UV光谱电化学法和理论计算所证明的。在最佳条件下(ACN + 0.10 M NBu 4 BF 4)进行本体电解可分别从4-硝基甲苯和4,4'-二硝基联苄中分离出40%和67%的E -4,4'-二硝基sti。


























 京公网安备 11010802027423号
京公网安备 11010802027423号