当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermophysical Properties of Imidazolium-Based Binary Ionic Liquid Mixtures Using Molecular Dynamics Simulations
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-03-19 , DOI: 10.1021/acs.jced.7b01028 Utkarsh Kapoor 1 , Jindal K. Shah 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-03-19 , DOI: 10.1021/acs.jced.7b01028 Utkarsh Kapoor 1 , Jindal K. Shah 1
Affiliation
|
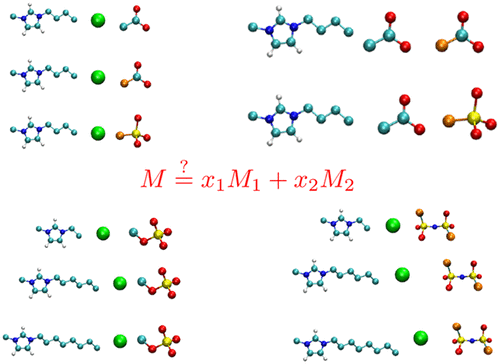
Until very recently, task-specific ionic liquids have been designed by altering the chemistry of either the cation, anion, or both. An alternative approach, that is gaining considerable momentum, is to consider ionic liquids that are derived from mixing two different ionic liquids and varying the molar composition of such blends to exert precise control over the desired physicochemical and biological properties. As the number of ionic liquids that result from mixing is projected to be close to a billion, it is highly desirable to predict a priori whether ionic liquid mixtures can be considered as ideal solutions of their pure analogues. Toward this end, we employ molecular dynamics simulations to predict the density, molar volumes, excess molar volumes, self-diffusion coefficients, and ionic conductivities for 11 ionic liquid mixture systems as a function of mole fractions spanning the entire range of compositions of the constituent ionic liquids. The ionic liquid mixtures investigated here are 1-n-butyl-3-methylimidazolium [C4mim]+ chloride Cl– paired with [C4mim]+ acetate [CH3COO]−/[OAC]−, [C4mim]+ trifluoroacetate [CF3COO]−/[TFA]− and [C4mim]+ trifluoromethanesulfonate [CF3SO3]−/[TFS]−, and [C4mim][OAC] combined with [C4mim][TFA] and [C4mim][TFS]. The effect of change in the alkyl chain length on the thermophysical properties of ionic liquid mixtures containing anions as Cl––methylsulfate [MeSO4]−, and Cl––bis(trifluoromethanesulfonyl)imide [NTf2]− is evaluated by coupling with 1-ethyl-3-methylimidazolium [C2mim]+, 1-n-hexyl-3-methylimidazolium [C6mim]+, and 1-n-octyl-3-methylimidazolium [C8mim]+ cations. The deviation of the property trend from the linear mixing rule is discussed in terms of the difference in the properties of pure ionic liquid analogues.
中文翻译:

咪唑基二元离子液体混合物的热力学性质的分子动力学模拟
直到最近,已经通过改变阳离子,阴离子或两者的化学性质来设计特定任务的离子液体。获得巨大动力的另一种方法是考虑将两种不同的离子液体混合并改变这种共混物的摩尔组成而得到的离子液体,以对所需的物理化学和生物学特性进行精确控制。由于混合产生的离子液体的数量预计将接近十亿,因此非常需要预测先验是否可以将离子液体混合物视为其纯类似物的理想溶液。为此,我们采用分子动力学模拟来预测11种离子液体混合物系统的密度,摩尔体积,过量摩尔体积,自扩散系数和离子电导率,其作为整个组分组成范围内摩尔分数的函数离子液体。此处研究的离子液体混合物是1-正丁基-3-甲基咪唑鎓[C 4 mim] +氯化物Cl –与[C 4 mim] +乙酸酯[CH 3 COO] - / [OAC] -配对,[C 4 mim ] +三氟乙酸盐[CF 3 COO] - / [TFA] -和[C 4 mim] +三氟甲磺酸盐[CF 3 SO 3 ] - / [TFS] -和[C 4 mim] [OAC]与[C 4 mim]结合[ TFA]和[C 4 mim] [TFS]。变化的在烷基链长度上含有阴离子例如Cl离子液体混合物的热物理性能的影响- -methylsulfate [内消旋4 ] -和Cl -双(三氟甲烷磺酰)亚胺[NTF 2 ] -通过与1-乙基-3-甲基咪唑鎓[C 2 mim] +,1 - n-己基-3-甲基咪唑鎓[C 6 mim] +和1 - n-辛基-3-甲基咪唑鎓[C 8 mim]偶联来评估+阳离子。根据纯离子液体类似物的特性差异,讨论了特性趋势与线性混合规则的偏差。
更新日期:2018-06-03
中文翻译:

咪唑基二元离子液体混合物的热力学性质的分子动力学模拟
直到最近,已经通过改变阳离子,阴离子或两者的化学性质来设计特定任务的离子液体。获得巨大动力的另一种方法是考虑将两种不同的离子液体混合并改变这种共混物的摩尔组成而得到的离子液体,以对所需的物理化学和生物学特性进行精确控制。由于混合产生的离子液体的数量预计将接近十亿,因此非常需要预测先验是否可以将离子液体混合物视为其纯类似物的理想溶液。为此,我们采用分子动力学模拟来预测11种离子液体混合物系统的密度,摩尔体积,过量摩尔体积,自扩散系数和离子电导率,其作为整个组分组成范围内摩尔分数的函数离子液体。此处研究的离子液体混合物是1-正丁基-3-甲基咪唑鎓[C 4 mim] +氯化物Cl –与[C 4 mim] +乙酸酯[CH 3 COO] - / [OAC] -配对,[C 4 mim ] +三氟乙酸盐[CF 3 COO] - / [TFA] -和[C 4 mim] +三氟甲磺酸盐[CF 3 SO 3 ] - / [TFS] -和[C 4 mim] [OAC]与[C 4 mim]结合[ TFA]和[C 4 mim] [TFS]。变化的在烷基链长度上含有阴离子例如Cl离子液体混合物的热物理性能的影响- -methylsulfate [内消旋4 ] -和Cl -双(三氟甲烷磺酰)亚胺[NTF 2 ] -通过与1-乙基-3-甲基咪唑鎓[C 2 mim] +,1 - n-己基-3-甲基咪唑鎓[C 6 mim] +和1 - n-辛基-3-甲基咪唑鎓[C 8 mim]偶联来评估+阳离子。根据纯离子液体类似物的特性差异,讨论了特性趋势与线性混合规则的偏差。


























 京公网安备 11010802027423号
京公网安备 11010802027423号