当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gold(i)-catalyzed diastereoselective synthesis of 1-α-oxybenzyl-1H-indenes†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8ob00406d Cintia Virumbrales 1, 2, 3, 4, 5 , Samuel Suárez-Pantiga 1, 2, 3, 4, 5 , Marta Solas 1, 2, 3, 4, 5 , Manuel A. Fernández-Rodríguez 1, 2, 3, 4, 5 , Roberto Sanz 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8ob00406d Cintia Virumbrales 1, 2, 3, 4, 5 , Samuel Suárez-Pantiga 1, 2, 3, 4, 5 , Marta Solas 1, 2, 3, 4, 5 , Manuel A. Fernández-Rodríguez 1, 2, 3, 4, 5 , Roberto Sanz 1, 2, 3, 4, 5
Affiliation
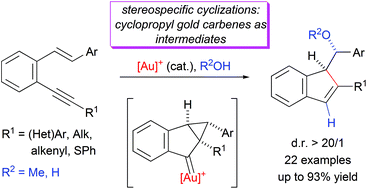
|
The gold(I)-catalyzed oxycyclization of β-aryl monosubstituted o-(alkynyl)styrenes gives rise to 1-α-methoxy or 1-α-hydroxybenzyl-1H-indenes in a diastereospecific way. In contrast to β,β-disubstituted o-(alkynyl)styrenes, the stereochemical outcome of this process, diastereospecific reaction supports the higher contribution of a gold intermediate with a cyclopropylcarbene-like character.
中文翻译:

金(i)催化1-α-氧基苄基-1 H-茚的非对映选择性合成†
金(I)催化的β-芳基单取代的邻-(炔基)苯乙烯的氧环化以非对映特异性的方式产生1-α-甲氧基或1-α-羟基苄基-1 H-茚。与该过程的立体化学结果-β,β-二取代的邻-(炔基)苯乙烯相反,非对映特异性反应支持具有环丙基卡宾样特征的金中间体的较高贡献。
更新日期:2018-03-19
中文翻译:

金(i)催化1-α-氧基苄基-1 H-茚的非对映选择性合成†
金(I)催化的β-芳基单取代的邻-(炔基)苯乙烯的氧环化以非对映特异性的方式产生1-α-甲氧基或1-α-羟基苄基-1 H-茚。与该过程的立体化学结果-β,β-二取代的邻-(炔基)苯乙烯相反,非对映特异性反应支持具有环丙基卡宾样特征的金中间体的较高贡献。


























 京公网安备 11010802027423号
京公网安备 11010802027423号