Molecular Catalysis ( IF 4.6 ) Pub Date : 2018-03-16 , DOI: 10.1016/j.mcat.2018.03.006 Tianyu Gao , Yongxuan Yin , Wenhao Fang , Qiue Cao
|
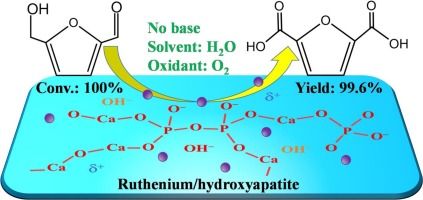
The aerobic oxidation of bio-platform compound 5-hydroxymethylfurfural (HMF) to value-added 2,5-furandicarboxylic acid (FDCA) was studied over hydroxyapatite supported ruthenium (Ru/HAP) nanocatalyst without extra addition of soluble base. Molecular oxygen and water were used as the oxidant and solvent, respectively. Various dominant reaction parameters, including HMF/Ru molar ratio, O2 pressure, reaction temperature and reaction time were systematically investigated. When HMF/Ru molar ratio was of 25, Ru/HAP catalyst exhibited the optimal catalytic performance, i.e., total conversion of HMF and 99.6% yield of FDCA were achieved at 120 °C and 1 MPa O2. Kinetic study revealed that the oxidation of HMF to FDCA followed a tandem pathway and the oxidation of carbonyl group in 5-formylfuran-2-carboxylic acid (FFCA) was found as the rate-determining step. Besides, well-dispersed uniformly-small metallic Ru0 nanoparticles and the acidic-basic sites presented on HAP surface were both essential to drive this base-free oxidation process. During the kinetic-controlled range (i.e., initial 1 h of reaction), the activity of the catalyst can be almost remained after five consecutive cycles; afterwards a minor decline in activity was observed mainly due to the partial oxidation of surface metallic Ru species, but the activity can be entirely recovered by a hydrogen reduction at 350 °C.
中文翻译:

羟基磷灰石上高度分散的钌纳米颗粒,作为选择性和可重复使用的催化剂,可在无碱条件下将5-羟甲基糠醛好氧氧化为2,5-呋喃二甲酸
在羟基磷灰石负载的钌(Ru / HAP)纳米催化剂上研究了生物平台化合物5-羟甲基糠醛(HMF)有氧氧化为增值的2,5-呋喃二甲酸(FDCA),而无需额外添加可溶性碱。分子氧和水分别用作氧化剂和溶剂。系统地研究了HMF / Ru摩尔比,O 2压力,反应温度和反应时间等主要反应参数。当HMF / Ru摩尔比为25时,Ru / HAP催化剂表现出最佳的催化性能,即HMF的总转化率和FDCA的总收率在120°C和1 MPa O 2下达到99.6%。。动力学研究表明,HMF向FDCA的氧化遵循串联途径,并且在5-甲酰基呋喃-2-甲酸(FFCA)中羰基的氧化被确定为速率决定步骤。此外,良好分散的均匀小金属Ru 0纳米颗粒和HAP表面上存在的酸性碱性位点对于驱动该无碱氧化过程都是必不可少的。在动力学控制的范围内(即反应的最初1小时),在连续五个循环后,催化剂的活性几乎可以保持不变。之后,观察到活性略有下降,主要是由于表面金属Ru物种的部分氧化,但是可以通过在350°C下进行氢还原来完全恢复活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号