Catalysis Today ( IF 5.3 ) Pub Date : 2018-03-17 , DOI: 10.1016/j.cattod.2018.03.030 Hui Liu , Fuli Yang , Bochao Yang , Qian Zhang , Yujun Chai , Ning Wang
|
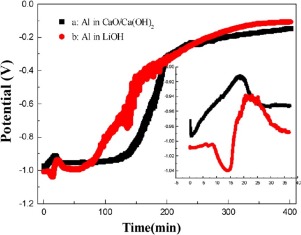
The effects of CaO and waste lithium foil on the aluminum-water reaction are investigated in this study. When CaO and waste lithium foil are added to deionized water, the pH of the solution rises above 12 and the Al–H2O reaction is noticeably affected. Two rapid hydrogen generation processes and a passivation process are observed in CaO/Ca(OH)2 suspension. A slow reaction follows the rapid hydrogen generation in LiOH solution. At the beginning of the process, the rapid reaction in CaO/Ca(OH)2 suspension and LiOH solution corresponds the rapid consumption of OH– ions. A similar tendency as the hydrogen generation process in CaO/Ca(OH)2 is detected by the in–situ open circuit potential. Solutions of LiOH and CaO/Ca(OH)2 initially present a difference in the rate of hydrogen generation, but they demonstrate a similar behavior after the consecutive addition of Al batches. This may be due to the synergistic reaction of OH– ions and the self-catalytic behavior of Al(OH)3, formed as a by product of the reaction.
中文翻译:

在碱溶液中通过铝-水反应快速产生氢
研究了CaO和废锂箔对铝-水反应的影响。当将CaO和废锂箔添加到去离子水中时,溶液的pH值升至12以上,并且Al–H 2 O反应受到明显影响。在CaO / Ca(OH)2悬浮液中观察到两个快速的氢生成过程和钝化过程。在LiOH溶液中快速生成氢气之后,反应缓慢。在过程的开始时,以CaO /钙的快速反应(OH)2悬浮液和LiOH溶液相当于OH的快速消耗-离子。与CaO / Ca(OH)2中的制氢过程相似的趋势通过原位开路电势检测。LiOH和CaO / Ca(OH)2的溶液最初呈现出氢气产生速率的差异,但是在连续添加Al批次后,它们表现出相似的行为。这可能是由于OH的协同反应-离子和Al(OH)的自催化行为3,通过该反应的产物形成为。


























 京公网安备 11010802027423号
京公网安备 11010802027423号