Biosensors and Bioelectronics ( IF 12.6 ) Pub Date : 2018-03-17 , DOI: 10.1016/j.bios.2018.03.034 Mahmoud Amouzadeh Tabrizi , Mojtaba Shamsipur , Reza Saber , Saeed Sarkar
|
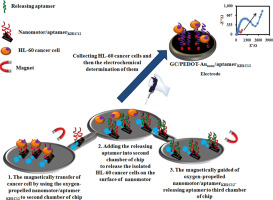
Herein, aptamer-modified self-propelled nanomotors were used for transportation of human promyelocytic leukemia cells (HL-60) from a human serum sample. For this purpose, the fabricated manganese oxide nanosheets-polyethyleneimine decorated with nickel/gold nanoparticles (MnO2-PEI/Ni/Au) as nanomotors were added to a vial containing thiolated aptamer KH1C12 solution as a capture aptamer to attach to the gold nanoparticles on the surface of nanomotors covalently. The aptamer-modified self-propelled nanomotors (aptamerKH1C12/nanomotors) were then separated by placing the vial in a magnetic stand. The aptamer-modified self-propelled nanomotors were rinsed three times with water to remove the non-attached aptamers. Then, the resulting aptamerKH1C12/nanomotors were applied for the on-the-fly” transporting of HL-60 cancer cell from a human serum sample. To release of the captured HL-60 cancer cells, the complementary nucleotide sequences of KH1C12 aptamer solution (releasing aptamer) that has a with capture aptamer was added to phosphate buffer solution (1 M, pH 7.4) containing HL-60/aptamerKH1C12/nanomotors. Because of the high affinity of capture aptamer to complementary nucleotide sequences of aptamerKH1C12, the HL-60 cancer cells released on the surface of aptamerKH1C12/nanomotors into the solution. The second goal of the present work was determining the concentration of HL-60 cancer cell in the human serum samples. The electrochemical impedance spectroscopy technique (EIS) was used for the determination of HL-60 cancer cell. The concentration of separated cancer cell was determined by aptamer/gold nanoparticles-poly(3,4-ethylene dioxythiophene) modified GC electrode (GC/PEDOT-Aunano/aptamer KH1C12). The proposed aptasensor exhibited a good response to the concentration of HL-60 cancer cells in the range of 2.5 × 101 to 5 × 105 cells mL-1 with a low limit of detection of 250 cells mL-1.
中文翻译:

MnO 2 -PEI / Ni / Au / aptamer作为新型纳米电机从人血清样品中分离HL-60癌细胞,并通过适配体/金纳米颗粒-聚(3,4-乙撑二氧噻吩)修饰的GC电极对其进行电化学测定
本文中,适体修饰的自推进纳米马达用于从人血清样品中运输人早幼粒细胞白血病细胞(HL-60)。为此,将装饰有镍/金纳米颗粒(MnO 2 -PEI / Ni / Au)作为纳米马达的氧化锰纳米片-聚乙烯亚胺添加到装有硫醇化适体KH1C12溶液作为捕获适体的小瓶中,以附着到纳米金上纳米马达的表面共价。然后通过将小瓶放在磁力架上分离适体修饰的自推进纳米马达(适体KH1C12 /纳米马达)。用水将适体修饰的自推进纳米马达冲洗三遍,以除去未连接的适体。然后,得到适体KH1C12纳米马达被用于从人血清样品中“快速”转运HL-60癌细胞。为了释放被捕获的HL-60癌细胞,将具有捕获适体的KH1C12适体溶液(释放适体)的互补核苷酸序列添加到含有HL-60 /适体KH1C12 /的磷酸盐缓冲液(1 M,pH 7.4)中。纳米马达。由于捕获适体与适体KH1C12的互补核苷酸序列具有很高的亲和力,因此HL-60癌细胞在适体KH1C12的表面上释放/ nanomotors解决方案。本工作的第二个目标是确定人血清样品中HL-60癌细胞的浓度。电化学阻抗谱技术(EIS)用于测定HL-60癌细胞。通过适体/金纳米颗粒-聚(3,4-乙撑二氧噻吩)修饰的GC电极(GC / PEDOT-Au纳米/适体KH1C12)确定分离的癌细胞的浓度。所提出的适体传感器对HL-60癌细胞的浓度范围为2.5×10 1至5×10 5个细胞mL -1表现出良好的响应,而对250个细胞mL -1的检测下限较低。


























 京公网安备 11010802027423号
京公网安备 11010802027423号