当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Au@Pd Bimetallic Nanocatalyst for Carbon–Halogen Bond Cleavage: An Old Story with New Insight into How the Activity of Pd is Influenced by Au
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1021/acs.est.7b05996 Rui Liu 1 , Hui-min Chen 1, 2 , Li-ping Fang 2 , Cuihong Xu 1, 2 , Zuoliang He 1 , Yujian Lai 1 , Huachao Zhao 1 , Deribachew Bekana 1 , Jing-fu Liu 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1021/acs.est.7b05996 Rui Liu 1 , Hui-min Chen 1, 2 , Li-ping Fang 2 , Cuihong Xu 1, 2 , Zuoliang He 1 , Yujian Lai 1 , Huachao Zhao 1 , Deribachew Bekana 1 , Jing-fu Liu 1
Affiliation
|
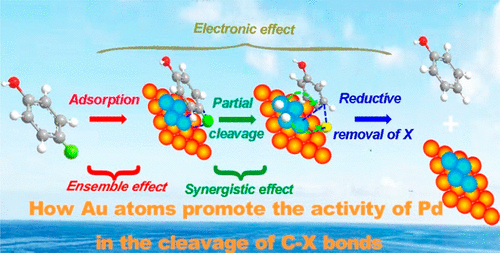
AuPd bimetallic nanocatalysts exhibit superior catalytic performance in the cleavage of carbon–halogen bonds (C–X) in the hazardous halogenated pollutants. A better understanding of how Au atoms promote the reactivity of Pd sites rather than vaguely interpreting as bimetallic effect and determining which type of Pd sites are necessary for these reactions are crucial factors for the design of atomically precise nanocatalysts that make full use of both the Pd and Au atoms. Herein, we systematically manipulated the coordination number of Pd–Pd, d-orbital occupation state, and the Au–Pd interface of the Pd reactive centers and studied the structure–activity relationship of Au–Pd in the catalyzed cleavage of C–X bonds. It is revealed that Au enhanced the activity of Pd atoms primarily by increasing the occupation state of Pd d-orbitals. Meanwhile, among the Pd sites formed on the Au surface, five to seven contiguous Pd atoms, three or four adjacent Pd atoms, and isolated Pd atoms were found to be the most active in the cleavage of C–Cl, C–Br, and C–I bonds, respectively. Besides, neighboring Au atoms directly contribute to the weakening of the C–Br/C–I bond. This work provides new insight into the rational design of bimetallic metal catalysts with specific catalytic properties.
中文翻译:

用于碳-卤素键裂解的Au @ Pd双金属纳米催化剂:一个古老的故事,对Au对Pd的活性有何影响的新见解
AuPd双金属纳米催化剂在有害卤代污染物中的碳-卤素键(C-X)裂解中表现出优异的催化性能。更好地了解Au原子如何促进Pd位的反应性,而不是模糊地解释为双金属效应并确定这些反应所必需的Pd位类型是设计原子级精确纳米催化剂的关键因素,该催化剂必须充分利用Pd和Pd两者。和金原子。在本文中,我们系统地操纵了Pd-Pd的配位数,d轨道占据状态和Pd反应中心的Au-Pd界面,并研究了Au-Pd在C-X键催化裂解中的结构-活性关系。 。揭示了Au主要通过增加Pd d-轨道的占据状态来增强Pd原子的活性。同时,在Au表面上形成的Pd位点中,发现五到七个连续的Pd原子,三个或四个相邻的Pd原子和孤立的Pd原子在C–Cl,C–Br和C–的裂解中最活跃。我分别绑定。此外,邻近的金原子直接导致C-Br / C-I键的削弱。这项工作为具有特定催化性能的双金属金属催化剂的合理设计提供了新的见识。
更新日期:2018-03-21
中文翻译:

用于碳-卤素键裂解的Au @ Pd双金属纳米催化剂:一个古老的故事,对Au对Pd的活性有何影响的新见解
AuPd双金属纳米催化剂在有害卤代污染物中的碳-卤素键(C-X)裂解中表现出优异的催化性能。更好地了解Au原子如何促进Pd位的反应性,而不是模糊地解释为双金属效应并确定这些反应所必需的Pd位类型是设计原子级精确纳米催化剂的关键因素,该催化剂必须充分利用Pd和Pd两者。和金原子。在本文中,我们系统地操纵了Pd-Pd的配位数,d轨道占据状态和Pd反应中心的Au-Pd界面,并研究了Au-Pd在C-X键催化裂解中的结构-活性关系。 。揭示了Au主要通过增加Pd d-轨道的占据状态来增强Pd原子的活性。同时,在Au表面上形成的Pd位点中,发现五到七个连续的Pd原子,三个或四个相邻的Pd原子和孤立的Pd原子在C–Cl,C–Br和C–的裂解中最活跃。我分别绑定。此外,邻近的金原子直接导致C-Br / C-I键的削弱。这项工作为具有特定催化性能的双金属金属催化剂的合理设计提供了新的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号