当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dynamic Characteristics of Guanosine-5′-monophosphate Reductase Complexes Revealed by High-Resolution 31P Field-Cycling NMR Relaxometry
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.biochem.8b00142 Masha M. Rosenberg 1 , Alfred G. Redfield 2 , Mary F. Roberts 3 , Lizbeth Hedstrom 1, 4
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.biochem.8b00142 Masha M. Rosenberg 1 , Alfred G. Redfield 2 , Mary F. Roberts 3 , Lizbeth Hedstrom 1, 4
Affiliation
|
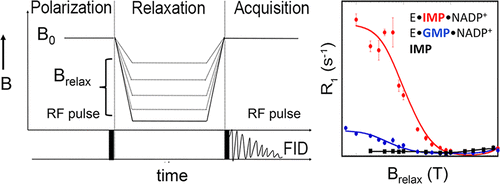
The ability of enzymes to modulate the dynamics of bound substrates and cofactors is a critical feature of catalysis, but the role of dynamics has largely been approached from the perspective of the protein. Here, we use an underappreciated NMR technique, subtesla high-resolution field-cycling 31P NMR relaxometry, to interrogate the dynamics of enzyme bound substrates and cofactors in guanosine-5′-monophosphate reductase (GMPR). These experiments reveal distinct binding modes and dynamic profiles associated with the 31P nuclei in the Michaelis complexes for the deamination and hydride transfer steps of the catalytic cycle. Importantly, the substrate is constrained and the cofactor is more dynamic in the deamination complex E·GMP·NADP+, whereas the substrate is more dynamic and the cofactor is constrained in the hydride transfer complex E·IMP·NADP+. The presence of D2O perturbed the relaxation of the 31P nuclei in E·IMP·NADP+ but not in E·GMP·NADP+, providing further evidence of distinct binding modes with different dynamic properties. dIMP and dGMP are poor substrates, and the dynamics of the cofactor complexes of dGMP/dIMP are disregulated relative to GMP/IMP. The substrate 2’-OH interacts with Asp219, and mutation of Asp219 to Ala decreases the value of Vmax by a factor of 30. Counterintuitively, loss of Asp219 makes both substrates and cofactors less dynamic. These observations suggest that the interactions between the substrate 2’-OH and Asp219 coordinate the dynamic properties of the Michaelis complexes, and these dynamics are important for progression through the catalytic cycle.
中文翻译:

高分辨率31 P场循环NMR弛豫法揭示鸟苷5'-单磷酸还原酶配合物的动态特性。
酶调节结合的底物和辅因子动力学的能力是催化的关键特征,但是从蛋白质的角度来看,动力学的作用已被广泛研究。在这里,我们使用低估的NMR技术,subtesla高分辨率场循环31 P NMR弛豫法,询问鸟苷5'-单磷酸还原酶(GMPR)中酶结合的底物和辅因子的动力学。这些实验揭示了与Michaelis配合物中31 P核相关的独特的结合模式和动态特性,用于催化循环的脱氨和氢化物转移步骤。重要的是,在脱氨基复合物E·GMP·NADP +中,底物受到约束,辅因子更动态。,而底物更具动态性,并且辅因子被限制在氢化物转移复合物E·IMP·NADP +中。D 2 O的存在扰乱了E·IMP·NADP +中31 P核的弛豫,但不干扰E·GMP·NADP +中31 P核的弛豫,为具有不同动态特性的独特结合模式提供了进一步的证据。dIMP和dGMP是较差的底物,相对于GMP / IMP,dGMP / dIMP的辅因子复合物的动力学是失调的。底物2'-OH与Asp219相互作用,并且Asp219突变为Ala降低了V max的值相差30倍。与直觉相反,Asp219的损失使底物和辅因子的动态性降低。这些观察结果表明,底物2'-OH和Asp219之间的相互作用协调了Michaelis配合物的动力学性质,并且这些动力学对于整个催化循环的进行是重要的。
更新日期:2018-03-16
中文翻译:

高分辨率31 P场循环NMR弛豫法揭示鸟苷5'-单磷酸还原酶配合物的动态特性。
酶调节结合的底物和辅因子动力学的能力是催化的关键特征,但是从蛋白质的角度来看,动力学的作用已被广泛研究。在这里,我们使用低估的NMR技术,subtesla高分辨率场循环31 P NMR弛豫法,询问鸟苷5'-单磷酸还原酶(GMPR)中酶结合的底物和辅因子的动力学。这些实验揭示了与Michaelis配合物中31 P核相关的独特的结合模式和动态特性,用于催化循环的脱氨和氢化物转移步骤。重要的是,在脱氨基复合物E·GMP·NADP +中,底物受到约束,辅因子更动态。,而底物更具动态性,并且辅因子被限制在氢化物转移复合物E·IMP·NADP +中。D 2 O的存在扰乱了E·IMP·NADP +中31 P核的弛豫,但不干扰E·GMP·NADP +中31 P核的弛豫,为具有不同动态特性的独特结合模式提供了进一步的证据。dIMP和dGMP是较差的底物,相对于GMP / IMP,dGMP / dIMP的辅因子复合物的动力学是失调的。底物2'-OH与Asp219相互作用,并且Asp219突变为Ala降低了V max的值相差30倍。与直觉相反,Asp219的损失使底物和辅因子的动态性降低。这些观察结果表明,底物2'-OH和Asp219之间的相互作用协调了Michaelis配合物的动力学性质,并且这些动力学对于整个催化循环的进行是重要的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号