当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fragment-Based Approach to Targeting Inosine-5'-monophosphate Dehydrogenase (IMPDH) from Mycobacterium tuberculosis.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-03-23 , DOI: 10.1021/acs.jmedchem.7b01622 Ana Trapero 1 , Angela Pacitto 2 , Vinayak Singh 3 , Mohamad Sabbah 1 , Anthony G Coyne 1 , Valerie Mizrahi 3 , Tom L Blundell 2 , David B Ascher 2, 4 , Chris Abell 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-03-23 , DOI: 10.1021/acs.jmedchem.7b01622 Ana Trapero 1 , Angela Pacitto 2 , Vinayak Singh 3 , Mohamad Sabbah 1 , Anthony G Coyne 1 , Valerie Mizrahi 3 , Tom L Blundell 2 , David B Ascher 2, 4 , Chris Abell 1
Affiliation
|
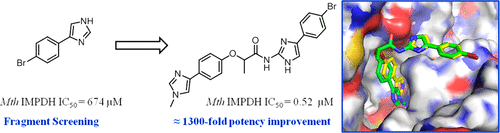
Tuberculosis (TB) remains a major cause of mortality worldwide, and improved treatments are needed to combat emergence of drug resistance. Inosine 5'-monophosphate dehydrogenase (IMPDH), a crucial enzyme required for de novo synthesis of guanine nucleotides, is an attractive TB drug target. Herein, we describe the identification of potent IMPDH inhibitors using fragment-based screening and structure-based design techniques. Screening of a fragment library for Mycobacterium thermoresistible ( Mth) IMPDH ΔCBS inhibitors identified a low affinity phenylimidazole derivative. X-ray crystallography of the Mth IMPDH ΔCBS-IMP-inhibitor complex revealed that two molecules of the fragment were bound in the NAD binding pocket of IMPDH. Linking the two molecules of the fragment afforded compounds with more than 1000-fold improvement in IMPDH affinity over the initial fragment hit.
中文翻译:

靶向结核分枝杆菌肌苷 5'-单磷酸脱氢酶 (IMPDH) 的基于片段的方法。
结核病 (TB) 仍然是全世界死亡的主要原因,需要改进治疗以对抗耐药性的出现。肌苷 5'-单磷酸脱氢酶 (IMPDH) 是从头合成鸟嘌呤核苷酸所需的关键酶,是一种有吸引力的结核病药物靶点。在这里,我们描述了使用基于片段的筛选和基于结构的设计技术来鉴定有效的 IMPDH 抑制剂。筛选耐热分枝杆菌 (Mth) IMPDH ΔCBS 抑制剂的片段库确定了一种低亲和力苯基咪唑衍生物。第 M 个 IMPDH ΔCBS-IMP-抑制剂复合物的 X 射线晶体学显示该片段的两个分子结合在 IMPDH 的 NAD 结合口袋中。
更新日期:2018-03-16
中文翻译:

靶向结核分枝杆菌肌苷 5'-单磷酸脱氢酶 (IMPDH) 的基于片段的方法。
结核病 (TB) 仍然是全世界死亡的主要原因,需要改进治疗以对抗耐药性的出现。肌苷 5'-单磷酸脱氢酶 (IMPDH) 是从头合成鸟嘌呤核苷酸所需的关键酶,是一种有吸引力的结核病药物靶点。在这里,我们描述了使用基于片段的筛选和基于结构的设计技术来鉴定有效的 IMPDH 抑制剂。筛选耐热分枝杆菌 (Mth) IMPDH ΔCBS 抑制剂的片段库确定了一种低亲和力苯基咪唑衍生物。第 M 个 IMPDH ΔCBS-IMP-抑制剂复合物的 X 射线晶体学显示该片段的两个分子结合在 IMPDH 的 NAD 结合口袋中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号