Separation and Purification Technology ( IF 8.6 ) Pub Date : 2018-03-16 , DOI: 10.1016/j.seppur.2018.03.030 Hicham Zazou , Nihal Oturan , Mutlu Sönmez Çelebi , Mohamed Hamdani , Mehmet A. Oturan
|
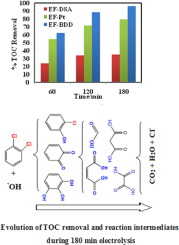
This study reports on the electrochemical degradation and mineralization of 1,2-dichlorobenzene, called also ortho-dichlorobenzene (o-DCB) in aqueous solution, using an undivided electrochemical reactor for the application of electro-Fenton (EF) process. The o-DCB was chosen as model pollutant because of its high toxicity and persistence in the environment. Among the different parameters influencing process efficiency, the effect of different anode materials such as DSA, BDD and Pt on the production of hydroxyl radicals (OH) was investigated. These radicals were generated concomitantly from the oxidation of water on the anode surface and from electrochemically generated Fenton’s reagent in bulk of solution. The effects of current, supporting electrolyte and the nature of the anode material on degradation kinetic and mineralization efficiency were studied. The absolute rate constant for oxidation of o-DCB by
OH was determined as (1.61 ± 0.02) × 109 M−1 s−1 by using the competition kinetic method. Mineralization of o-DCB aqueous solution was monitored using total organic carbon (TOC) analysis. Under optimum conditions, i.e., 500 mA current, BDD anode and CF cathode, treatment of o-DCB solution containing 50 mM Na2SO4 and 0.1 mM Fe2+ at pH = 3.0 conducted to more than 90% TOC removal in 3 h electrolysis. Oxidative degradation products formed during the treatment (aromatic intermediates and short-chain carboxylic acids) or end-products (inorganic ions) were identified and quantified by using HPLC and ion chromatography techniques respectively, and based on the identified products a degradation pathway for mineralization of o-DCB was proposed.
中文翻译:

使用DSA /碳毡,Pt /碳毡和BDD /碳毡池通过电化学高级氧化对水溶液中的1,2-二氯苯进行冷焚烧
这项研究报告了使用未分式电化学反应器进行电芬顿(EF)工艺应用的水溶液中1,2-二氯苯(也称为邻二氯苯(o- DCB))的电化学降解和矿化作用。的邻- DCB被选为模型污染物由于在环境中它的高毒性和持久性。在影响工艺效率的不同参数中,不同的阳极材料(例如DSA,BDD和Pt)对羟基自由基产生的影响(OH)进行了调查。这些自由基是由阳极表面的水氧化和大量溶液中电化学产生的芬顿试剂伴随产生的。研究了电流,支持电解质和阳极材料的性质对降解动力学和矿化效率的影响。绝对速率常数的氧化ö通过-DCB
OH被确定为(1.61±0.02)×10 9中号-1小号-1通过使用竞争动力学方法。使用总有机碳(TOC)分析监测邻-DCB水溶液的矿化。在最佳条件下,即500 mA电流,BDD阳极和CF阴极,对o进行处理在3小时的电解过程中,将含有50 mM Na 2 SO 4和0.1 mM Fe 2+的pH = 3.0的-DCB溶液进行TOC去除率超过90%。分别通过HPLC和离子色谱技术鉴定和量化处理过程中形成的氧化降解产物(芳族中间体和短链羧酸)或终产物(无机离子),并基于鉴定出的产物,确定氧化降解产物的矿化途径。Ø -DCB提出。


























 京公网安备 11010802027423号
京公网安备 11010802027423号