当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility of Ionic Liquid [Bmim]Ac in Supercritical CO2 Containing Different Cosolvents
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.jced.7b01108 Gen Li 1 , Dan Zhou 1 , Qin-Qin Xu 1 , Guo-Yue Qiao 1 , Jian-Zhong Yin 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.jced.7b01108 Gen Li 1 , Dan Zhou 1 , Qin-Qin Xu 1 , Guo-Yue Qiao 1 , Jian-Zhong Yin 1
Affiliation
|
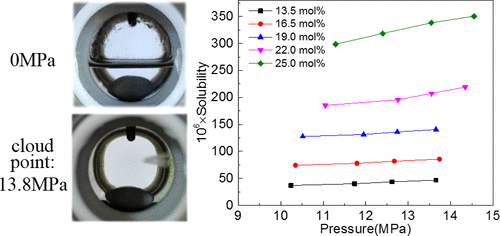
The solubility of the ionic liquid (IL) 1-butyl-3-methylimidazolium acetate ([Bmim]Ac) in supercritical carbon dioxide (scCO2) with cosolvents, including ethanol, acetone, dimethyl sulfoxide (DMSO), and acetonitrile, was determined at 40, 50, and 60 °C and with a pressure up to 15.0 MPa. The results showed that the addition of cosolvents has a significant effect on the solubility of [Bmim]Ac in scCO2. The ability of different cosolvents to enhance the solubility of [Bmim]Ac in scCO2 is in the following order: ethanol > DMSO > acetone > acetonitrile. The solubility of [Bmim]Ac in the scCO2/cosolvent mixture increased dramatically as the cosolvent concentration exceeded 20.0 mol %. The effect of the temperature on the solubility is more complicated. The solubility of [Bmim]Ac in scCO2/cosolvent increased slowly when using ethanol as the cosolvent, decreased slowly when using acetone or acetonitrile as the cosolvent, and increased first and then decreased when using DMSO as the cosolvent as the temperature increased from 40 to 60 °C. Moreover, the values of solubility increased as the pressure increased from 8 to 15 MPa. The increased tendency in the high pressure area is more obvious. The maximum solubility is 3.66 × 10–2 mol %, which can be obtained when using 26.0 mol % ethanol at 60 °C, 14.55 MPa, and the minimum solubility is 1.89 × 10–4 mol %, which can be obtained when using 10.5 mol % DMSO at 40 °C, 9.93 MPa. The modified Christal equation was used to correlate the solubility data, and the average absolute relative deviations are in the range of 4.36–14.35%. The maximum correlation accuracy is obtained when using ethanol as the cosolvent, and the minimum value for the system is obtained when using DMSO as the cosolvent.
中文翻译:

离子液体[Bmim] Ac在含有不同助溶剂的超临界CO 2中的溶解度
确定了离子液体(IL)乙酸1-丁基-3-甲基咪唑鎓盐([Bmim] Ac)在超临界二氧化碳(scCO 2)中与包括乙醇,丙酮,二甲基亚砜(DMSO)和乙腈在内的助溶剂的溶解度温度为40、50和60°C,压力最高为15.0 MPa。结果表明,助溶剂的加入对[Bmim] Ac在scCO 2中的溶解度具有显着影响。不同助溶剂增强[Bmim] Ac在scCO 2中的溶解度的能力依次为:乙醇> DMSO>丙酮>乙腈。[Bmim] Ac在scCO 2中的溶解度当助溶剂浓度超过20.0 mol%时,/助溶剂混合物急剧增加。温度对溶解度的影响更为复杂。当使用乙醇作为助溶剂时,[Bmim] Ac在scCO 2 /助溶剂中的溶解度缓慢增加,当使用丙酮或乙腈作为助溶剂时,其溶解度缓慢降低,而当使用DMSO作为助溶剂时,随着温度从40升高,其溶解度先升高然后降低。到60°C。此外,随着压力从8 MPa增加到15 MPa,溶解度值增加。高压区的增加趋势更加明显。最大溶解度为3.66×10 –2 mol%,在60°C,14.55 MPa下使用26.0 mol%乙醇时可获得,最低溶解度为1.89×10 –4mol%,当在40℃,9.93MPa下使用10.5mol%的DMSO时可获得。修改后的Christal方程用于关联溶解度数据,平均绝对相对偏差在4.36–14.35%的范围内。当使用乙醇作为助溶剂时,可获得最大的相关精度,而当使用DMSO作为助溶剂时,可获得系统的最小值。
更新日期:2018-03-16
中文翻译:

离子液体[Bmim] Ac在含有不同助溶剂的超临界CO 2中的溶解度
确定了离子液体(IL)乙酸1-丁基-3-甲基咪唑鎓盐([Bmim] Ac)在超临界二氧化碳(scCO 2)中与包括乙醇,丙酮,二甲基亚砜(DMSO)和乙腈在内的助溶剂的溶解度温度为40、50和60°C,压力最高为15.0 MPa。结果表明,助溶剂的加入对[Bmim] Ac在scCO 2中的溶解度具有显着影响。不同助溶剂增强[Bmim] Ac在scCO 2中的溶解度的能力依次为:乙醇> DMSO>丙酮>乙腈。[Bmim] Ac在scCO 2中的溶解度当助溶剂浓度超过20.0 mol%时,/助溶剂混合物急剧增加。温度对溶解度的影响更为复杂。当使用乙醇作为助溶剂时,[Bmim] Ac在scCO 2 /助溶剂中的溶解度缓慢增加,当使用丙酮或乙腈作为助溶剂时,其溶解度缓慢降低,而当使用DMSO作为助溶剂时,随着温度从40升高,其溶解度先升高然后降低。到60°C。此外,随着压力从8 MPa增加到15 MPa,溶解度值增加。高压区的增加趋势更加明显。最大溶解度为3.66×10 –2 mol%,在60°C,14.55 MPa下使用26.0 mol%乙醇时可获得,最低溶解度为1.89×10 –4mol%,当在40℃,9.93MPa下使用10.5mol%的DMSO时可获得。修改后的Christal方程用于关联溶解度数据,平均绝对相对偏差在4.36–14.35%的范围内。当使用乙醇作为助溶剂时,可获得最大的相关精度,而当使用DMSO作为助溶剂时,可获得系统的最小值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号