当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
“Newly diagnosed Hepatocellular Carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study”
Journal of Hepatology ( IF 25.7 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.jhep.2018.03.009 Antonietta Romano , Paolo Angeli , Sara Piovesan , Franco Noventa , Georgios Anastassopoulos , Liliana Chemello , Luisa Cavalletto , Martina Gambato , Francesco Paolo Russo , Patrizia Burra , Valter Vincenzi , Pier Giorgio Scotton , Sandro Panese , Diego Tempesta , Tosca Bertin , Maurizio Carrara , Antonio Carlotto , Franco Capra , Giada Carolo , Giovanna Scroccaro , Alfredo Alberti
Journal of Hepatology ( IF 25.7 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.jhep.2018.03.009 Antonietta Romano , Paolo Angeli , Sara Piovesan , Franco Noventa , Georgios Anastassopoulos , Liliana Chemello , Luisa Cavalletto , Martina Gambato , Francesco Paolo Russo , Patrizia Burra , Valter Vincenzi , Pier Giorgio Scotton , Sandro Panese , Diego Tempesta , Tosca Bertin , Maurizio Carrara , Antonio Carlotto , Franco Capra , Giada Carolo , Giovanna Scroccaro , Alfredo Alberti
|
BACKGROUND & AIMS
Direct-acting antiviral agents (DAAs) are safe and effective in patients with hepatitis C. Conflicting data were reported on the risk of hepatocellular carcinoma (HCC) during/after therapy with DAAs. The aim of this study was to evaluate the incidence of newly diagnosed HCC and associated risk factors in patients with advanced hepatitis C treated with DAAs. METHODS
The study is based on the NAVIGATORE platform, a prospectively recording database of all patients with hepatitis C receiving DAAs in the Veneto region of Italy. The inclusion criteria were: fibrosis stage ≥F3. The exclusion criteria were: Child-Turcotte-Pugh (CTP)-C, liver transplantation before DAAs, history or presence of HCC, follow-up <4 weeks after starting DAAs. A total of 3,917 out of 4,234 consecutive patients were included, with a mean follow-up of 536.2 ± 197.6 days. RESULTS
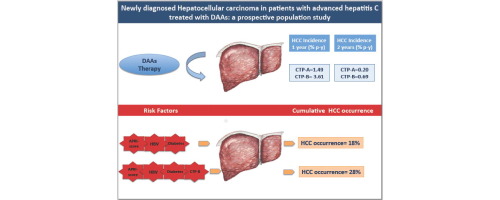
Overall, HCC was diagnosed in 55 patients. During the first year, HCC incidence was 0.46% (95% CI 0.12-1.17) in F3, 1.49% (1.03-2.08) in CTP-A and 3.61% (1.86-6.31) in CTP-B cirrhotics; in the second year, HCC incidences were 0%, 0.2%, and 0.69%, respectively. By multivariate analysis, HCC was significantly associated with an aspartate aminotransferase to platelet ratio ≥2.5 (hazard ratio [HR] 2.03; 95% CI 1.14-3.61; p = 0.016) and hepatitis B virus infection (HR 3.99; 1.24-12.91; p = 0.021). Failure to achieve a sustained virological response was strongly associated with development of HCC (HR 9.09; 5.2-16.1; p = 0.0001). A total of 29% of patients with HCC had an aggressive tumor, often seen in the early phase of treatment. CONCLUSIONS
These data, obtained in a large, prospective, population-based study, indicate that in patients with advanced hepatitis C receiving DAAs, the risk of "de novo" hepatocarcinoma during the first year is not higher, and might be lower, than that of untreated patients. The risk further declines thereafter. Early hepatocarcinoma appearance may reflect pre-existing, microscopic, undetectable tumors. LAY SUMMARY
Hepatocellular carcinoma is one of the complications of hepatitis C related cirrhosis. Treating patients with advanced hepatitis C with the new interferon-free direct-acting antiviral agents has been associated with improvement in liver function and survival, while more conflicting data have been reported regarding the risk of hepatocellular carcinoma. We report the results of a prospective population study on the incidence of newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with direct-acting antiviral agents, clearly indicating that the residual hepatocellular carcinoma risk is reduced and declines progressively with time after a sustained virological response. Development of a liver tumor during/after therapy was associated with known risk factors and with virological failure.
中文翻译:

“接受 DAA 治疗的晚期丙型肝炎患者新诊断出的肝细胞癌:一项前瞻性人群研究”
背景和目的 直接作用抗病毒药物 (DAA) 在丙型肝炎患者中是安全有效的。关于 DAA 治疗期间/之后发生肝细胞癌 (HCC) 风险的报告数据相互矛盾。本研究的目的是评估接受 DAA 治疗的晚期丙型肝炎患者新诊断 HCC 的发生率和相关危险因素。方法 该研究基于 NAVIGATORE 平台,该平台是意大利威尼托地区接受 DAA 的所有丙型肝炎患者的前瞻性记录数据库。纳入标准为:纤维化分期≥F3。排除标准为:Child-Turcotte-Pugh (CTP)-C、DAAs 前肝移植、HCC 病史或存在、开始 DAAs 后<4 周的随访。共纳入 4,234 名连续患者中的 3,917 名,平均随访 536 名。2 ± 197.6 天。结果 总体而言,55 名患者被诊断为 HCC。在第一年,F3 中 HCC 的发生率为 0.46%(95% CI 0.12-1.17),CTP-A 中为 1.49%(1.03-2.08),CTP-B 肝硬化中为 3.61%(1.86-6.31);第二年,HCC 的发生率分别为 0%、0.2% 和 0.69%。通过多变量分析,HCC 与天冬氨酸转氨酶与血小板比率≥2.5(风险比 [HR] 2.03;95% CI 1.14-3.61;p = 0.016)和乙型肝炎病毒感染(HR 3.99;1.24-12.91;p)显着相关= 0.021)。未能实现持续的病毒学应答与 HCC 的发展密切相关(HR 9.09;5.2-16.1;p = 0.0001)。共有 29% 的 HCC 患者患有侵袭性肿瘤,这通常出现在治疗的早期阶段。结论 这些数据是在一项大型、前瞻性、基于人群的研究中获得的,表明在接受 DAA 治疗的晚期丙型肝炎患者中,第一年“新发”肝癌的风险并不高于未经治疗的患者,甚至可能更低。此后风险进一步下降。早期肝癌外观可能反映了预先存在的、微观的、无法检测到的肿瘤。概述 肝细胞癌是丙型肝炎相关肝硬化的并发症之一。使用新的无干扰素直接作用抗病毒药物治疗晚期丙型肝炎患者与改善肝功能和存活率有关,而关于肝细胞癌风险的报道则存在更多相互矛盾的数据。我们报告了一项关于接受直接作用抗病毒药物治疗的晚期丙型肝炎患者新诊断肝细胞癌发病率的前瞻性人群研究结果,清楚地表明残留肝细胞癌风险在持续病毒学检查后随着时间的推移而降低并逐渐下降。回复。治疗期间/治疗后肝脏肿瘤的发展与已知的危险因素和病毒学失败有关。
更新日期:2018-08-01
中文翻译:

“接受 DAA 治疗的晚期丙型肝炎患者新诊断出的肝细胞癌:一项前瞻性人群研究”
背景和目的 直接作用抗病毒药物 (DAA) 在丙型肝炎患者中是安全有效的。关于 DAA 治疗期间/之后发生肝细胞癌 (HCC) 风险的报告数据相互矛盾。本研究的目的是评估接受 DAA 治疗的晚期丙型肝炎患者新诊断 HCC 的发生率和相关危险因素。方法 该研究基于 NAVIGATORE 平台,该平台是意大利威尼托地区接受 DAA 的所有丙型肝炎患者的前瞻性记录数据库。纳入标准为:纤维化分期≥F3。排除标准为:Child-Turcotte-Pugh (CTP)-C、DAAs 前肝移植、HCC 病史或存在、开始 DAAs 后<4 周的随访。共纳入 4,234 名连续患者中的 3,917 名,平均随访 536 名。2 ± 197.6 天。结果 总体而言,55 名患者被诊断为 HCC。在第一年,F3 中 HCC 的发生率为 0.46%(95% CI 0.12-1.17),CTP-A 中为 1.49%(1.03-2.08),CTP-B 肝硬化中为 3.61%(1.86-6.31);第二年,HCC 的发生率分别为 0%、0.2% 和 0.69%。通过多变量分析,HCC 与天冬氨酸转氨酶与血小板比率≥2.5(风险比 [HR] 2.03;95% CI 1.14-3.61;p = 0.016)和乙型肝炎病毒感染(HR 3.99;1.24-12.91;p)显着相关= 0.021)。未能实现持续的病毒学应答与 HCC 的发展密切相关(HR 9.09;5.2-16.1;p = 0.0001)。共有 29% 的 HCC 患者患有侵袭性肿瘤,这通常出现在治疗的早期阶段。结论 这些数据是在一项大型、前瞻性、基于人群的研究中获得的,表明在接受 DAA 治疗的晚期丙型肝炎患者中,第一年“新发”肝癌的风险并不高于未经治疗的患者,甚至可能更低。此后风险进一步下降。早期肝癌外观可能反映了预先存在的、微观的、无法检测到的肿瘤。概述 肝细胞癌是丙型肝炎相关肝硬化的并发症之一。使用新的无干扰素直接作用抗病毒药物治疗晚期丙型肝炎患者与改善肝功能和存活率有关,而关于肝细胞癌风险的报道则存在更多相互矛盾的数据。我们报告了一项关于接受直接作用抗病毒药物治疗的晚期丙型肝炎患者新诊断肝细胞癌发病率的前瞻性人群研究结果,清楚地表明残留肝细胞癌风险在持续病毒学检查后随着时间的推移而降低并逐渐下降。回复。治疗期间/治疗后肝脏肿瘤的发展与已知的危险因素和病毒学失败有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号