当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Macrophage-mediated delivery of light activated nitric oxide prodrugs with spatial, temporal and concentration control†
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1039/c8sc00015h Michael A. Evans 1, 2, 3, 4, 5 , Po-Ju Huang 1, 2, 3, 4 , Yuji Iwamoto 1, 6, 7, 8 , Kelly N. Ibsen 2, 3, 4, 5, 9 , Emory M. Chan 10, 11, 12, 13 , Yutaka Hitomi 1, 6, 7, 8 , Peter C. Ford 1, 2, 3, 4 , Samir Mitragotri 2, 3, 4, 5, 9
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1039/c8sc00015h Michael A. Evans 1, 2, 3, 4, 5 , Po-Ju Huang 1, 2, 3, 4 , Yuji Iwamoto 1, 6, 7, 8 , Kelly N. Ibsen 2, 3, 4, 5, 9 , Emory M. Chan 10, 11, 12, 13 , Yutaka Hitomi 1, 6, 7, 8 , Peter C. Ford 1, 2, 3, 4 , Samir Mitragotri 2, 3, 4, 5, 9
Affiliation
|
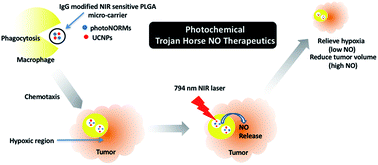
Nitric oxide (NO) holds great promise as a treatment for cancer hypoxia, if its concentration and localization can be precisely controlled. Here, we report a “Trojan Horse” strategy to provide the necessary spatial, temporal, and dosage control of such drug-delivery therapies at targeted tissues. Described is a unique package consisting of (1) a manganese–nitrosyl complex, which is a photoactivated NO-releasing moiety (photoNORM), plus Nd3+-doped upconverting nanoparticles (Nd-UCNPs) incorporated into (2) biodegradable polymer microparticles that are taken up by (3) bone-marrow derived murine macrophages. Both the photoNORM [Mn(NO)dpaqNO2]BPh4(dpaqNO2 = 2-[N,N-bis(pyridin-2-yl-methyl)]-amino-N′-5-nitro-quinolin-8-yl-acetamido) and the Nd-UCNPs are activated by tissue-penetrating near-infrared (NIR) light at ∼800 nm. Thus, simultaneous therapeutic NO delivery and photoluminescence (PL) imaging can be achieved with a NIR diode laser source. The loaded microparticles are non-toxic to their macrophage hosts in the absence of light. The microparticle-carrying macrophages deeply penetrate into NIH-3T3/4T1 tumor spheroid models, and when the infiltrated spheroids are irradiated with NIR light, NO is released in quantifiable amounts while emission from the Nd-UCNPs provides images of microparticle location. Furthermore, varying the intensity of the NIR excitation allows photochemical control over NO release. Low doses reduce levels of hypoxia inducible factor 1 alpha (HIF-1α) in the tumor cells, while high doses are cytotoxic. The use of macrophages to carry microparticles with a NIR photo-activated theranostic payload into a tumor overcomes challenges often faced with therapeutic administration of NO and offers the potential of multiple treatment strategies with a single system.
中文翻译:

巨噬细胞介导的光活化一氧化氮前药的递送,具有空间,时间和浓度控制功能†
如果可以精确控制其浓度和位置,一氧化氮(NO)有望作为治疗癌症缺氧的方法。在这里,我们报告“特洛伊木马”策略,以在目标组织上提供此类药物递送疗法的必要空间,时间和剂量控制。描述了一个独特的包装,该包装包括(1)锰-亚硝酰基配合物,它是光活化的NO释放部分(photoNORM),以及掺入(2)可生物降解的聚合物微粒中的Nd 3+掺杂的上转换纳米颗粒(Nd-UCNP)。被(3)骨髓来源的鼠巨噬细胞吸收。都是photoNORM [Mn(NO)dpaq NO 2 ] BPh 4(dpaq NO 2 = 2- [ N,N-双(吡啶-2-基-甲基)]-氨基-N(5-硝基-喹啉-8-乙酰氨基)和Nd-UCNPs被组织穿透的〜800 nm的近红外(NIR)光激活。因此,可以使用NIR二极管激光源实现同时的治疗性NO输送和光致发光(PL)成像。在没有光照的情况下,负载的微粒对其巨噬细胞宿主无毒。携带微粒的巨噬细胞深深地渗透到NIH-3T3 / 4T1肿瘤球体模型中,当用NIR光照射渗透的球体时,NO以可定量释放,而Nd-UCNPs的发射则提供了微粒位置的图像。此外,改变NIR激发的强度可以对NO的释放进行光化学控制。低剂量可降低肿瘤细胞中缺氧诱导因子1α(HIF-1α)的水平,而高剂量则具有细胞毒性。
更新日期:2018-03-16
中文翻译:

巨噬细胞介导的光活化一氧化氮前药的递送,具有空间,时间和浓度控制功能†
如果可以精确控制其浓度和位置,一氧化氮(NO)有望作为治疗癌症缺氧的方法。在这里,我们报告“特洛伊木马”策略,以在目标组织上提供此类药物递送疗法的必要空间,时间和剂量控制。描述了一个独特的包装,该包装包括(1)锰-亚硝酰基配合物,它是光活化的NO释放部分(photoNORM),以及掺入(2)可生物降解的聚合物微粒中的Nd 3+掺杂的上转换纳米颗粒(Nd-UCNP)。被(3)骨髓来源的鼠巨噬细胞吸收。都是photoNORM [Mn(NO)dpaq NO 2 ] BPh 4(dpaq NO 2 = 2- [ N,N-双(吡啶-2-基-甲基)]-氨基-N(5-硝基-喹啉-8-乙酰氨基)和Nd-UCNPs被组织穿透的〜800 nm的近红外(NIR)光激活。因此,可以使用NIR二极管激光源实现同时的治疗性NO输送和光致发光(PL)成像。在没有光照的情况下,负载的微粒对其巨噬细胞宿主无毒。携带微粒的巨噬细胞深深地渗透到NIH-3T3 / 4T1肿瘤球体模型中,当用NIR光照射渗透的球体时,NO以可定量释放,而Nd-UCNPs的发射则提供了微粒位置的图像。此外,改变NIR激发的强度可以对NO的释放进行光化学控制。低剂量可降低肿瘤细胞中缺氧诱导因子1α(HIF-1α)的水平,而高剂量则具有细胞毒性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号