当前位置:
X-MOL 学术
›
J. Anal. Appl. Pyrol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
THERMAL CRACKING OF N-BUTYLBENZENE AT HIGH PRESSURE: EXPERIMENTAL STUDY AND KINETIC MODELLING
Journal of Analytical and Applied Pyrolysis ( IF 6 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.jaap.2018.03.016 N.C. Leguizamon Guerra , J.C. Lizardo Huerta , C. Lorgeoux , R. Michels , R. Fournet , B. Sirjean , A. Randi , R. Bounaceur , V. Burklé-Vitzthum
Journal of Analytical and Applied Pyrolysis ( IF 6 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.jaap.2018.03.016 N.C. Leguizamon Guerra , J.C. Lizardo Huerta , C. Lorgeoux , R. Michels , R. Fournet , B. Sirjean , A. Randi , R. Bounaceur , V. Burklé-Vitzthum
|
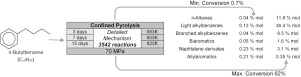
Abstract The thermal cracking of n-butylbenzene was experimentally studied at high pressure (70 MPa), and moderate temperatures (583, 603, 623 K), for conversions of n-butylbenzene ranging between 0.7% and 62%. The pyrolysis was performed in sealed isobaric gold tubes (confined pyrolysis). Three main chemical families were observed: short alkylbenzenes (mostly toluene and ethylbenzene), branched alkylbenzenes (isomers of iso-butylbenzene and iso-heptylbenzene) and short alkanes (from CH4 to C4H10). As minor products, alkenylbenzenes (styrene and butenylbenzene), methylindane and biaromatic structures were also quantified. A detailed kinetic model composed of 3542 free-radical reactions and 383 species (molecules and free-radicals) was written in a systematic manner by taking into account all relevant elementary free-radical reactions. A large number of thermochemical and kinetic parameters were computed by theoretical calculations. A very good agreement between experimental and simulation results is observed for every operating condition and for most major and minor compounds. The apparent kinetic parameters were computed at 623 K, 70 MPa and 30% conversion under the assumption of a first-order global rate law: the apparent activation energy was found equal to 66.6 kcal mol−1 and the frequency factor to 6.3 × 1016 s−1. The extrapolation to low temperature (473 K), which is characteristic of deeply buried oil reservoirs, shows that the stability of n-butylbenzene is about the same as the stability of alkanes, but n-butylbenzene is more stable than n-decylbenzene and less stable than toluene.
中文翻译:

正丁苯在高压下的热裂解:实验研究和动力学建模
摘要 在高压 (70 MPa) 和中等温度 (583, 603, 623 K) 下,对正丁苯的热裂解进行了实验研究,正丁苯的转化率在 0.7% 到 62% 之间。热解在密封的等压金管中进行(密闭热解)。观察到三个主要化学家族:短烷基苯(主要是甲苯和乙苯)、支链烷基苯(异丁基苯和异庚基苯的异构体)和短链烷烃(从 CH4 到 C4H10)。作为次要产物,烯基苯(苯乙烯和丁烯基苯)、甲基茚满和双芳烃结构也被量化。通过考虑所有相关的基本自由基反应,以系统的方式编写了由 3542 个自由基反应和 383 个物种(分子和自由基)组成的详细动力学模型。通过理论计算计算了大量的热化学和动力学参数。对于每种操作条件以及大多数主要和次要化合物,都观察到实验和模拟结果之间的非常好的一致性。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)的外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。对于每种操作条件以及大多数主要和次要化合物,都观察到实验和模拟结果之间的非常好的一致性。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)的外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。对于每种操作条件以及大多数主要和次要化合物,都观察到实验和模拟结果之间的非常好的一致性。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)的外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)的外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。
更新日期:2018-08-01
中文翻译:

正丁苯在高压下的热裂解:实验研究和动力学建模
摘要 在高压 (70 MPa) 和中等温度 (583, 603, 623 K) 下,对正丁苯的热裂解进行了实验研究,正丁苯的转化率在 0.7% 到 62% 之间。热解在密封的等压金管中进行(密闭热解)。观察到三个主要化学家族:短烷基苯(主要是甲苯和乙苯)、支链烷基苯(异丁基苯和异庚基苯的异构体)和短链烷烃(从 CH4 到 C4H10)。作为次要产物,烯基苯(苯乙烯和丁烯基苯)、甲基茚满和双芳烃结构也被量化。通过考虑所有相关的基本自由基反应,以系统的方式编写了由 3542 个自由基反应和 383 个物种(分子和自由基)组成的详细动力学模型。通过理论计算计算了大量的热化学和动力学参数。对于每种操作条件以及大多数主要和次要化合物,都观察到实验和模拟结果之间的非常好的一致性。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)的外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。对于每种操作条件以及大多数主要和次要化合物,都观察到实验和模拟结果之间的非常好的一致性。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)的外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。对于每种操作条件以及大多数主要和次要化合物,都观察到实验和模拟结果之间的非常好的一致性。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)的外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)的外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。表观动力学参数是在 623 K、70 MPa 和 30% 转化率下计算的,假设一阶全局速率定律:发现表观活化能等于 66.6 kcal mol-1,频率因子为 6.3 × 1016 s -1。对深埋油藏特征的低温(473 K)外推表明,正丁苯的稳定性与烷烃的稳定性大致相同,但正丁苯比正癸苯更稳定,而比甲苯稳定。



























 京公网安备 11010802027423号
京公网安备 11010802027423号