European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.ejmech.2018.03.034 Jui-Yin Yu , Hsiu-Jung Cheng , Huei-Ru Wu , Wei-Shen Wu , Jui-Wen Lu , Ting-Jen Cheng , Ying-Ta Wu , Jim-Min Fang
|
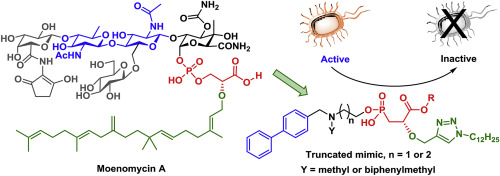
Transglycosylase (TGase) is essential to biosynthesis of peptidoglycan for formation of bacterial cell wall. Moenomycin is a potent TGase inhibitor, but not used in clinic treatment due to its poor pharmacokinetics. The E−F disaccharide, phosphoglycerate and lipid tail in moenomycin are crucial elements for TGase inhibition and antibacterial activity. Based on this scaffold, a series of truncated mimics comprising biphenyl, amine linker and 2-alkoxy-3-phosphorylpropanoate moieties were designed to test their TGase inhibitory activity. In this design, the phosphorylpropanoate group is a surrogate of phosphoglycerate with improved stability. A library of lipid tails can be constructed by a straightforward approach using Cu(I)-catalyzed (3 + 2) cycloaddition reactions, and the as-synthesized triazole ring can provide additional hydrogen bonds in the TGase active site. Our molecular docking experiments reveal that the biphenyl group provides π–π and π–cation interactions to act as a simplified alternative of the C–E disaccharide in moenomycin. To play the role of the oxonium transition state in transglycosylation, the amine linker exists as a positively charged species in physiological condition to attain electrostatic interactions with acidic residues. In this study, two biphenyl-linked 2-alkoxy-3-phosphorylpropanoate compounds (8 and 10) are found to exhibit modest inhibitory activity (IC50 ≈ 150 μM) against the TGase of Acinetobacter baumannii and good antibacterial activity against Staphylococcus aureus (MIC = 6.3 μM).
中文翻译:

结合联苯,胺连接基和2-烷氧基-3-磷酸基丙酸酯部分的细菌转糖基化酶抑制剂的基于结构的设计
转糖基化酶(TGase)对肽聚糖生物合成以形成细菌细胞壁至关重要。Moenomycin是一种有效的TGase抑制剂,但由于其药代动力学较差,因此未用于临床治疗。Moenomycin中的E-F二糖,磷酸甘油酸酯和脂质尾巴是TGase抑制和抗菌活性的关键元素。基于此支架,设计了一系列包含联苯,胺连接基和2-烷氧基-3-磷酸基丙酸酯的截短模拟物,以测试其TGase抑制活性。在该设计中,磷酸酰基丙酸酯基团是磷酸甘油酸酯的替代物,具有改善的稳定性。可以使用Cu(I)催化的(3 + 2)环加成反应通过直接方法构建脂质尾巴库,合成的三唑环可以在TGase活性位点提供额外的氢键。我们的分子对接实验表明,联苯基团提供π–π和π–阳离子相互作用,可以作为莫诺霉素中C–E二糖的简化替代品。为了在氧化糖基化中发挥氧鎓过渡态的作用,胺接头在生理条件下以带正电荷的形式存在,以实现与酸性残基的静电相互作用。在这项研究中,有两种联苯连接的2-烷氧基-3-磷酰基丙酸酯化合物(胺接头在生理条件下以带正电荷的形式存在,以实现与酸性残基的静电相互作用。在这项研究中,有两种联苯连接的2-烷氧基-3-磷酰基丙酸酯化合物(胺接头在生理条件下以带正电荷的形式存在,以实现与酸性残基的静电相互作用。在这项研究中,有两种联苯连接的2-烷氧基-3-磷酰基丙酸酯化合物(8和10)被发现表现出适度的抑制活性(IC 50 ≈150μM)对的转谷氨酰胺酶鲍曼不动杆菌和良好的抗菌活性对金黄色葡萄球菌(MIC = 6.3μM)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号