Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
DNA Conformation Induces Adaptable Binding by Tandem Zinc Finger Proteins.
Cell ( IF 64.5 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.cell.2018.02.058 Anamika Patel 1 , Peng Yang 2 , Matthew Tinkham 2 , Mihika Pradhan 1 , Ming-An Sun 2 , Yixuan Wang 2 , Don Hoang 2 , Gernot Wolf 2 , John R Horton 3 , Xing Zhang 3 , Todd Macfarlan 2 , Xiaodong Cheng 4
Cell ( IF 64.5 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.cell.2018.02.058 Anamika Patel 1 , Peng Yang 2 , Matthew Tinkham 2 , Mihika Pradhan 1 , Ming-An Sun 2 , Yixuan Wang 2 , Don Hoang 2 , Gernot Wolf 2 , John R Horton 3 , Xing Zhang 3 , Todd Macfarlan 2 , Xiaodong Cheng 4
Affiliation
|
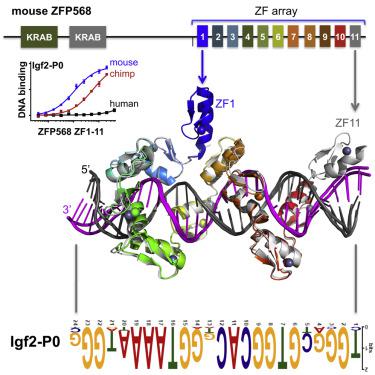
Tandem zinc finger (ZF) proteins are the largest and most rapidly diverging family of DNA-binding transcription regulators in mammals. ZFP568 represses a transcript of placental-specific insulin like growth factor 2 (Igf2-P0) in mice. ZFP568 binds a 24-base pair sequence-specific element upstream of Igf2-P0 via the eleven-ZF array. Both DNA and protein conformations deviate from the conventional one finger-three bases recognition, with individual ZFs contacting 2, 3, or 4 bases and recognizing thymine on the opposite strand. These interactions arise from a shortened minor groove caused by an AT-rich stretch, suggesting adaptability of ZF arrays to sequence variations. Despite conservation in mammals, mutations at Igf2 and ZFP568 reduce their binding affinity in chimpanzee and humans. Our studies provide important insights into the evolutionary and structural dynamics of ZF-DNA interactions that play a key role in mammalian development and evolution.
中文翻译:

DNA构象通过串联锌指蛋白诱导适应性结合。
串联锌指(ZF)蛋白是哺乳动物中最大的,发展最快的DNA结合转录调节子家族。ZFP568在小鼠中抑制胎盘特异性胰岛素样生长因子2(Igf2-P0)的转录物。ZFP568通过11-ZF阵列结合Igf2-P0上游的24个碱基对的序列特异性元件。DNA和蛋白质构象均偏离常规的一指三碱基识别,单个ZF接触2、3或4个碱基并识别相反链上的胸腺嘧啶。这些相互作用是由于富含AT的拉伸引起的短沟的缩短而产生的,这表明ZF阵列对序列变异的适应性。尽管在哺乳动物中有所保护,但Igf2和ZFP568的突变降低了它们对黑猩猩和人类的结合亲和力。
更新日期:2018-03-16
中文翻译:

DNA构象通过串联锌指蛋白诱导适应性结合。
串联锌指(ZF)蛋白是哺乳动物中最大的,发展最快的DNA结合转录调节子家族。ZFP568在小鼠中抑制胎盘特异性胰岛素样生长因子2(Igf2-P0)的转录物。ZFP568通过11-ZF阵列结合Igf2-P0上游的24个碱基对的序列特异性元件。DNA和蛋白质构象均偏离常规的一指三碱基识别,单个ZF接触2、3或4个碱基并识别相反链上的胸腺嘧啶。这些相互作用是由于富含AT的拉伸引起的短沟的缩短而产生的,这表明ZF阵列对序列变异的适应性。尽管在哺乳动物中有所保护,但Igf2和ZFP568的突变降低了它们对黑猩猩和人类的结合亲和力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号