当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The mechanism of catalase loading into porous vaterite CaCO3 crystals by co-synthesis†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c7cp07836f A. S. Vikulina 1, 2, 3, 4 , N. A. Feoktistova 5, 6, 7, 8, 9 , N. G. Balabushevich 5, 6, 7, 8 , A. G. Skirtach 10, 11, 12, 13 , D. Volodkin 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c7cp07836f A. S. Vikulina 1, 2, 3, 4 , N. A. Feoktistova 5, 6, 7, 8, 9 , N. G. Balabushevich 5, 6, 7, 8 , A. G. Skirtach 10, 11, 12, 13 , D. Volodkin 1, 2, 3, 4, 5
Affiliation
|
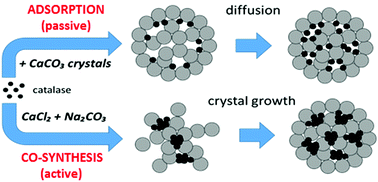
Porous vaterite CaCO3 crystals are nowadays extensively used as high-capacity bio-friendly sacrificial templates for the fabrication of such protein-containing nano- and micro-particles as capsules and beads. The first step in the protein encapsulation is performed through loading of the protein molecules into the crystals. Co-synthesis is one of the most useful and simple methods proven to effectively load crystals with proteins; however, the loading mechanism is still unknown. To understand the mechanism, in this study, we focus on the loading of a model protein catalase into the crystals by means of adsorption into pre-formed crystals (ADS) and co-synthesis (COS). Analysis of the physico-chemical characteristics of the protein in solution and during the loading and simulation of the protein packing into the crystals are performed. COS provides more effective loading than ADS giving protein contents in the crystals of 20.3 and 3.5 w/w%, respectively. Extremely high loading for COS providing a local protein concentration of about 550 mg mL−1 is explained by intermolecular protein interactions, i.e. formation of protein aggregates induced by CaCl2 during the co-synthesis. This is supported by a lower equilibrium constant obtained for COS (5 × 105 M−1) than for ADS (23 × 105 M−1), indicating a higher affinity of single protein molecules rather than aggregates to the crystal surface. Fitting the adsorption isotherms by classical adsorption models has shown that the Langmuir and BET models describe the adsorption phenomenon better than the Freundlich model, proving the aggregation in solution followed by adsorption of the aggregates into the crystals. We believe that this study will be useful for protein encapsulation through CaCO3 crystals using the COS method.
中文翻译:

共合成法 将过氧化氢酶加载到多孔球ate石CaCO 3晶体中的机制†
多孔球ate石CaCO 3如今,晶体被广泛用作高容量生物友好的牺牲模板,用于制造诸如胶囊和珠子之类的含蛋白质的纳米和微粒。蛋白质封装的第一步是通过将蛋白质分子加载到晶体中来执行的。共合成是最有用和最简单的方法之一,已被证明可以有效地将蛋白质加载到晶体中。但是,加载机制仍然未知。为了了解机理,在这项研究中,我们着重于通过吸附到预先形成的晶体(ADS)和共合成(COS)中的方式将模型蛋白过氧化氢酶装载到晶体中。进行了溶液中蛋白质的物理化学特性分析以及在蛋白质加载和模拟过程中蛋白质向晶体中的填充过程。COS比ADS提供更有效的负载,使晶体中的蛋白质含量分别为20.3和3.5 w / w%。COS的极高负载可提供约550 mg mL的局部蛋白质浓度-1通过分子间蛋白质相互作用来解释,即在共合成过程中由CaCl 2诱导的蛋白质聚集体的形成。COS(5×10 5 M -1)低于ADS(23×10 5 M -1)所获得的平衡常数对此提供了支持。),表明单个蛋白质分子具有更高的亲和力,而不是聚集到晶体表面。用经典吸附模型拟合吸附等温线表明,Langmuir和BET模型比Freundlich模型描述的吸附现象更好,证明了溶液中的聚集,然后聚集体被吸附到晶体中。我们认为,这项研究对于使用COS方法通过CaCO 3晶体进行蛋白质包封将是有用的。
更新日期:2018-03-15
中文翻译:

共合成法 将过氧化氢酶加载到多孔球ate石CaCO 3晶体中的机制†
多孔球ate石CaCO 3如今,晶体被广泛用作高容量生物友好的牺牲模板,用于制造诸如胶囊和珠子之类的含蛋白质的纳米和微粒。蛋白质封装的第一步是通过将蛋白质分子加载到晶体中来执行的。共合成是最有用和最简单的方法之一,已被证明可以有效地将蛋白质加载到晶体中。但是,加载机制仍然未知。为了了解机理,在这项研究中,我们着重于通过吸附到预先形成的晶体(ADS)和共合成(COS)中的方式将模型蛋白过氧化氢酶装载到晶体中。进行了溶液中蛋白质的物理化学特性分析以及在蛋白质加载和模拟过程中蛋白质向晶体中的填充过程。COS比ADS提供更有效的负载,使晶体中的蛋白质含量分别为20.3和3.5 w / w%。COS的极高负载可提供约550 mg mL的局部蛋白质浓度-1通过分子间蛋白质相互作用来解释,即在共合成过程中由CaCl 2诱导的蛋白质聚集体的形成。COS(5×10 5 M -1)低于ADS(23×10 5 M -1)所获得的平衡常数对此提供了支持。),表明单个蛋白质分子具有更高的亲和力,而不是聚集到晶体表面。用经典吸附模型拟合吸附等温线表明,Langmuir和BET模型比Freundlich模型描述的吸附现象更好,证明了溶液中的聚集,然后聚集体被吸附到晶体中。我们认为,这项研究对于使用COS方法通过CaCO 3晶体进行蛋白质包封将是有用的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号