Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.bmcl.2018.03.002 Travis R Helgren 1 , Elif S Seven 1 , Congling Chen 1 , Thomas E Edwards 2 , Bart L Staker 3 , Jan Abendroth 2 , Peter J Myler 3 , James R Horn 1 , Timothy J Hagen 1
|
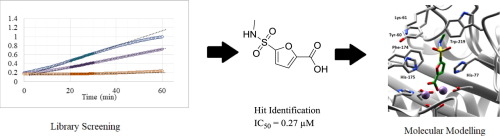
Methionine aminopeptidase (MetAP) is a dinuclear metalloprotease responsible for the cleavage of methionine initiator residues from nascent proteins. MetAP activity is necessary for bacterial proliferation and is therefore a projected novel antibacterial target. A compound library consisting of 294 members containing metal-binding functional groups was screened against Rickettsia prowazekii MetAP to determine potential inhibitory motifs. The compounds were first screened against the target at a concentration of 10 µM and potential hits were determined to be those exhibiting greater than 50% inhibition of enzymatic activity. These hit compounds were then rescreened against the target in 8-point dose–response curves and 11 compounds were found to inhibit enzymatic activity with IC50 values of less than 10 µM. Finally, compounds (1–5) were docked against RpMetAP with AutoDock to determine potential binding mechanisms and the results were compared with crystal structures deposited within the PDB.
中文翻译:

普瓦泽基立克次体甲硫氨酸氨基肽酶抑制化合物的鉴定用于抗菌应用
甲硫氨酸氨基肽酶 (MetAP) 是一种双核金属蛋白酶,负责从新生蛋白质中裂解甲硫氨酸起始残基。MetAP 活性对于细菌增殖是必需的,因此是预计的新型抗菌靶点。针对普氏立克次体MetAP筛选了由 294 个含有金属结合官能团的成员组成的化合物库,以确定潜在的抑制基序。首先以 10 µM 的浓度针对靶标筛选化合物,并确定潜在的命中是那些对酶活性抑制超过 50% 的化合物。然后根据 8 点剂量反应曲线中的靶标对这些命中化合物进行重新筛选,发现 11 种化合物可抑制酶活性,IC 50值小于 10 µM。最后,使用 AutoDock 将化合物 ( 1–5 ) 与Rp MetAP对接,以确定潜在的结合机制,并将结果与沉积在 PDB 内的晶体结构进行比较。



























 京公网安备 11010802027423号
京公网安备 11010802027423号