当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Arylsilylation of aryl halides using the magnetically recyclable bimetallic Pd–Pt–Fe3O4 catalyst †
Chemical Communications ( IF 4.9 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c7cc09926f Jisun Jang 1, 2, 3, 4 , Sangmoon Byun 1, 4, 5, 6, 7 , B. Moon Kim 1, 4, 5, 6, 7 , Sunwoo Lee 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c7cc09926f Jisun Jang 1, 2, 3, 4 , Sangmoon Byun 1, 4, 5, 6, 7 , B. Moon Kim 1, 4, 5, 6, 7 , Sunwoo Lee 1, 2, 3, 4
Affiliation
|
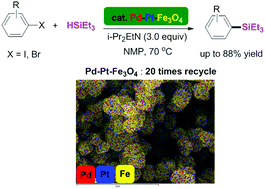
Transition metal-catalyzed silylations have typically involved the use of homogeneous non-recyclable catalytic systems. In this work, the first example of a recyclable catalytic system for the synthesis of arylsilanes has been reported, which utilizes the bimetallic complex, Pd–Pt–Fe3O4 nanoparticles. Various arylsilanes were prepared by the reaction of aryl iodides (or bromides) with hydrosilanes. This methodology showed good functional group tolerance toward ester, ketone, aldehyde, nitro, and cyano groups. The bimetallic Pd–Pt–Fe3O4 catalytic system showed better activity than monometallic Pt–Fe3O4 and Pd–Fe3O4 catalysts. In addition, the bimetallic Pd–Pt–Fe3O4 catalytic system could be easily recovered and reused for over twenty cycles.
中文翻译:

使用可磁循环使用的双金属Pd–Pt–Fe 3 O 4催化剂对卤代芳基进行芳基硅烷化†
过渡金属催化的甲硅烷基化通常涉及均相不可回收的催化体系的使用。在这项工作中,已经报道了合成芳基硅烷的可回收催化体系的第一个实例,该体系利用了双金属配合物Pd–Pt–Fe 3 O 4纳米颗粒。通过使芳基碘化物(或溴化物)与氢化硅烷反应来制备各种芳基硅烷。该方法显示出对酯,酮,醛,硝基和氰基的良好官能团耐受性。双金属Pd–Pt–Fe 3 O 4催化体系的活性优于单金属Pt–Fe 3 O 4和Pd–Fe 3 O 4。催化剂。此外,双金属Pd–Pt–Fe 3 O 4催化体系可以轻松回收并重复使用超过20个循环。
更新日期:2018-03-14
中文翻译:

使用可磁循环使用的双金属Pd–Pt–Fe 3 O 4催化剂对卤代芳基进行芳基硅烷化†
过渡金属催化的甲硅烷基化通常涉及均相不可回收的催化体系的使用。在这项工作中,已经报道了合成芳基硅烷的可回收催化体系的第一个实例,该体系利用了双金属配合物Pd–Pt–Fe 3 O 4纳米颗粒。通过使芳基碘化物(或溴化物)与氢化硅烷反应来制备各种芳基硅烷。该方法显示出对酯,酮,醛,硝基和氰基的良好官能团耐受性。双金属Pd–Pt–Fe 3 O 4催化体系的活性优于单金属Pt–Fe 3 O 4和Pd–Fe 3 O 4。催化剂。此外,双金属Pd–Pt–Fe 3 O 4催化体系可以轻松回收并重复使用超过20个循环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号