当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
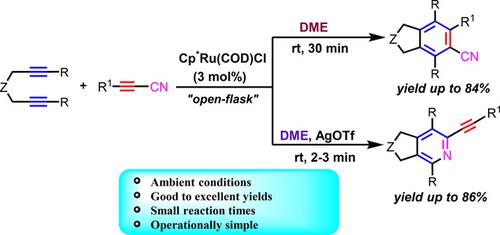
Additive‐Controlled Switchable Selectivity from Cyanobenzenes to 2‐Alkynylpyridines: Ruthenium(II)‐Catalyzed [2+2+2] Cycloadditions of Diynes and Alkynylnitriles
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-04-10 , DOI: 10.1002/adsc.201800228 Divya Bhatt 1 , Neha Patel 2 , Hrishikesh Chowdhury 1 , Prasad V. Bharatam 2 , Avijit Goswami 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-04-10 , DOI: 10.1002/adsc.201800228 Divya Bhatt 1 , Neha Patel 2 , Hrishikesh Chowdhury 1 , Prasad V. Bharatam 2 , Avijit Goswami 1
Affiliation
|
A highly efficient additive‐dependent chemoselective protocol for the synthesis of fused cyanoarenes and 2‐alkynylpyridines has been developed by the reaction of 1,6‐diynes with alkynylnitriles using chloro(pentamethylcyclopentadienyl) (cyclooctadiyne)ruthenium(II) as catalyst in dimethoxyethane (DME). The course of the reaction can be drastically altered simply by adding a catalytic amount of AgOTf as an additive resulting in a comprehensive shift in product formation from cyanoarenes to 2‐alkynylpyridines. Theoretical studies clearly indicate that the neutral Ru‐complex is responsible for the formation of cyanobenzenes, whereas the in situ generated cationic Ru‐complex plays a crucial role in the 2‐alkynylpyridines formation.
中文翻译:

氰基苯对2-炔基吡啶的加成控制可切换选择性:钌(II)催化的二炔和炔腈的[2 + 2 + 2]环加成反应
1,6-二炔与炔腈的反应通过使用氯(五甲基环戊二烯基)(环辛二炔)钌(II)作为二甲氧基乙烷(DME)的催化剂,已开发出一种高效的依赖添加剂的化学选择方案,用于合成稠合的氰基芳烃和2-炔基吡啶)。只需添加催化量的AgOTf作为添加剂,即可彻底改变反应过程,从而导致产物形成从氰基芳烃向2-炔基吡啶的全面转变。理论研究清楚地表明,中性Ru-络合物是氰基苯形成的原因,而原位生成的阳离子Ru-络合物在2-炔基吡啶的形成中起着至关重要的作用。
更新日期:2018-04-10
中文翻译:

氰基苯对2-炔基吡啶的加成控制可切换选择性:钌(II)催化的二炔和炔腈的[2 + 2 + 2]环加成反应
1,6-二炔与炔腈的反应通过使用氯(五甲基环戊二烯基)(环辛二炔)钌(II)作为二甲氧基乙烷(DME)的催化剂,已开发出一种高效的依赖添加剂的化学选择方案,用于合成稠合的氰基芳烃和2-炔基吡啶)。只需添加催化量的AgOTf作为添加剂,即可彻底改变反应过程,从而导致产物形成从氰基芳烃向2-炔基吡啶的全面转变。理论研究清楚地表明,中性Ru-络合物是氰基苯形成的原因,而原位生成的阳离子Ru-络合物在2-炔基吡啶的形成中起着至关重要的作用。

























 京公网安备 11010802027423号
京公网安备 11010802027423号