Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Urea on Solvation Dynamics and Rotational Relaxation of Coumarin 480 in Aqueous Micelles of Cationic Gemini Surfactants with Different Spacer Groups.
ACS Omega ( IF 4.1 ) Pub Date : 2018-03-14 , DOI: 10.1021/acsomega.7b01747 Sunita Kumari 1 , Sonu 1 , Sayantan Halder 1 , Rishika Aggrawal 1 , Ganapathisubramanian Sundar 2 , Subit K Saha 1
ACS Omega ( IF 4.1 ) Pub Date : 2018-03-14 , DOI: 10.1021/acsomega.7b01747 Sunita Kumari 1 , Sonu 1 , Sayantan Halder 1 , Rishika Aggrawal 1 , Ganapathisubramanian Sundar 2 , Subit K Saha 1
Affiliation
|
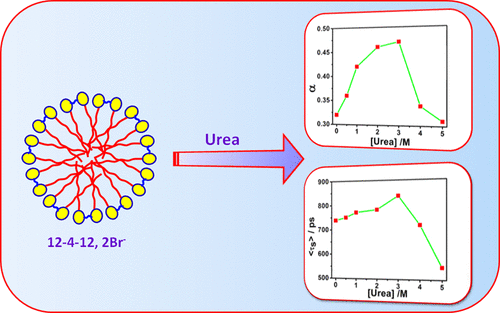
The present work highlights the effect of urea on solvation dynamics and the rotational relaxation of Coumarin 480 (C-480) in the Stern layer of aqueous micelles of cationic gemini surfactants, 12-4(OH) n -12 (n = 0, 1, 2). UV-visible absorption, steady-state fluorescence and fluorescence anisotropy, time-resolved fluorescence and fluorescence anisotropy, and dynamic light scattering measurements have been carried out for this study. The formation of micelles becomes disfavored in the presence of urea at high concentration. Solvation dynamics is bimodal in nature with fast solvation as a major component. The average solvation time increases, reaches a maximum, and then decreases with increasing concentration of urea because the degree of counterion dissociation also follows the same order with the addition of urea in the micellar solution. With increased degree of counterion dissociation, the extent of clustering of water molecules is increased, resulting in slower solvation process. The -OH group present in the spacer group of gemini surfactant controls the rate of solvation by shielding the water molecules from the probe molecules forming hydrogen bond. The microviscosity of micelles is decreased with increasing concentration of urea, as a result of which the rotational relaxation process becomes faster. In the presence of the -OH group in the spacer group, the microviscosity of micelles is enhanced, resulting in longer rotational relaxation time. Rotational relaxation process is bimodal in nature with the major contribution from the fast component to the fluorescence depolarization. Slow rotational relaxation is mainly due to the lateral diffusion of C-480 molecules along the surface of the micelle. The tumbling motion of the micelle as a whole is much slower than the lateral diffusion of C-480. Wobbling motion of C-480 becomes faster with increasing concentration of urea as a result of decreased microviscosity of micelles. The alignment of C-480 molecules in micelles might change with changing microviscosity.
中文翻译:

尿素对不同间隔基阳离子双子表面活性剂水溶液胶束中香豆素480溶解动力学和旋转弛豫的影响。
本工作着重指出了尿素对阳离子双子表面活性剂12-4(OH)n -12(n = 0,1)的水性胶束斯特恩层中香豆素480(C-480)的溶剂化动力学和旋转弛豫的影响。 ,2)。这项研究已经进行了紫外可见吸收,稳态荧光和荧光各向异性,时间分辨荧光和荧光各向异性以及动态光散射测量。在高浓度的尿素存在下,胶束的形成变得不利。溶剂化动力学本质上是双峰的,以快速溶剂化为主要成分。平均溶剂化时间增加,达到最大值,然后随着尿素浓度的增加而减少,这是因为抗衡离子的解离程度也遵循相同的顺序,即在胶束溶液中添加尿素。随着抗衡离子解离度的增加,水分子的聚集程度增加,导致溶剂化过程变慢。双子表面活性剂的间隔基中存在的-OH基团通过将水分子与形成氢键的探针分子屏蔽开,从而控制溶剂化速率。胶束的微粘度随着尿素浓度的增加而降低,其结果是旋转弛豫过程变得更快。在间隔基团中存在-OH基团时,胶束的微粘度提高,导致更长的旋转弛豫时间。旋转弛豫过程本质上是双峰的,其主要成分是快速组分对荧光去极化。缓慢的旋转弛豫主要是由于C-480分子沿胶束表面的横向扩散所致。胶束整体的翻滚运动比C-480的横向扩散慢得多。由于胶束的微粘度降低,随着尿素浓度的增加,C-480的摆动运动变得更快。胶束中C-480分子的排列可能随微粘度的变化而变化。
更新日期:2018-03-14
中文翻译:

尿素对不同间隔基阳离子双子表面活性剂水溶液胶束中香豆素480溶解动力学和旋转弛豫的影响。
本工作着重指出了尿素对阳离子双子表面活性剂12-4(OH)n -12(n = 0,1)的水性胶束斯特恩层中香豆素480(C-480)的溶剂化动力学和旋转弛豫的影响。 ,2)。这项研究已经进行了紫外可见吸收,稳态荧光和荧光各向异性,时间分辨荧光和荧光各向异性以及动态光散射测量。在高浓度的尿素存在下,胶束的形成变得不利。溶剂化动力学本质上是双峰的,以快速溶剂化为主要成分。平均溶剂化时间增加,达到最大值,然后随着尿素浓度的增加而减少,这是因为抗衡离子的解离程度也遵循相同的顺序,即在胶束溶液中添加尿素。随着抗衡离子解离度的增加,水分子的聚集程度增加,导致溶剂化过程变慢。双子表面活性剂的间隔基中存在的-OH基团通过将水分子与形成氢键的探针分子屏蔽开,从而控制溶剂化速率。胶束的微粘度随着尿素浓度的增加而降低,其结果是旋转弛豫过程变得更快。在间隔基团中存在-OH基团时,胶束的微粘度提高,导致更长的旋转弛豫时间。旋转弛豫过程本质上是双峰的,其主要成分是快速组分对荧光去极化。缓慢的旋转弛豫主要是由于C-480分子沿胶束表面的横向扩散所致。胶束整体的翻滚运动比C-480的横向扩散慢得多。由于胶束的微粘度降低,随着尿素浓度的增加,C-480的摆动运动变得更快。胶束中C-480分子的排列可能随微粘度的变化而变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号