当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Calcium Sensing by Recoverin: Effect of Protein Conformation on Ion Affinity
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jpclett.8b00495 Štěpán Timr 1 , Jan Kadlec 1 , Pavel Srb 1 , O. H. Samuli Ollila 1, 2 , Pavel Jungwirth 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jpclett.8b00495 Štěpán Timr 1 , Jan Kadlec 1 , Pavel Srb 1 , O. H. Samuli Ollila 1, 2 , Pavel Jungwirth 1
Affiliation
|
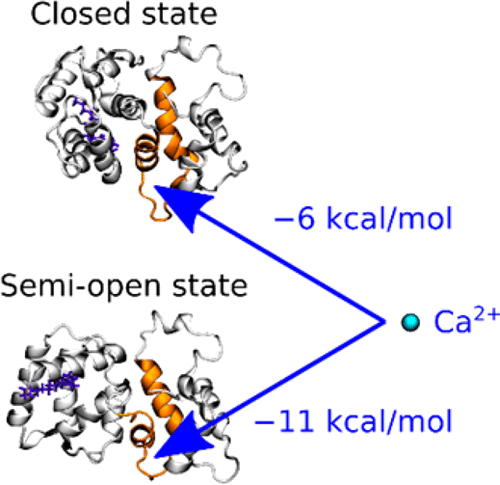
The detailed functional mechanism of recoverin, which acts as a myristoyl switch at the rod outer-segment disk membrane, is elucidated by direct and replica-exchange molecular dynamics. In accord with NMR structural evidence and calcium binding assays, simulations point to the key role of enhanced calcium binding to the EF3 loop of the semiopen state of recoverin as compared to the closed state. This 2–4-order decrease in calcium dissociation constant stabilizes the semiopen state in response to the increase of cytosolic calcium concentration in the vicinity of recoverin. A second calcium ion then binds to the EF2 loop and, consequently, the structure of the protein changes from the semiopen to the open state. The latter has the myristoyl chain extruded to the cytosol, ready to act as a membrane anchor of recoverin.
中文翻译:

恢复蛋白对钙的感应:蛋白质构象对离子亲和力的影响
直接和复制-交换分子动力学阐明了recoverin的详细功能机制,该机制在杆外节盘膜上充当肉豆蔻酰开关。与NMR结构证据和钙结合测定一致,模拟表明与闭合状态相比,钙与Recoveryin半开放状态的EF3环结合增强的关键作用。钙解离常数的这种2至4阶下降可稳定半开放状态,以响应于coverin附近胞质钙浓度的增加。然后,第二个钙离子与EF2环结合,因此,蛋白质的结构从半开放状态变为开放状态。后者的肉豆蔻酰链被挤出到细胞质中,准备充当recoverin的膜锚。
更新日期:2018-03-14
中文翻译:

恢复蛋白对钙的感应:蛋白质构象对离子亲和力的影响
直接和复制-交换分子动力学阐明了recoverin的详细功能机制,该机制在杆外节盘膜上充当肉豆蔻酰开关。与NMR结构证据和钙结合测定一致,模拟表明与闭合状态相比,钙与Recoveryin半开放状态的EF3环结合增强的关键作用。钙解离常数的这种2至4阶下降可稳定半开放状态,以响应于coverin附近胞质钙浓度的增加。然后,第二个钙离子与EF2环结合,因此,蛋白质的结构从半开放状态变为开放状态。后者的肉豆蔻酰链被挤出到细胞质中,准备充当recoverin的膜锚。


























 京公网安备 11010802027423号
京公网安备 11010802027423号