当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Consecutive Ring Expansion and Contraction for the Synthesis of 1‐Aryl Tetrahydroisoquinolines and Tetrahydrobenzazepines from Readily Available Heterocyclic Precursors
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201802188 Jessica E. Hill 1 , Johnathan V. Matlock 1 , Quentin Lefebvre 1 , Katie G. Cooper 2 , Jonathan Clayden 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201802188 Jessica E. Hill 1 , Johnathan V. Matlock 1 , Quentin Lefebvre 1 , Katie G. Cooper 2 , Jonathan Clayden 1
Affiliation
|
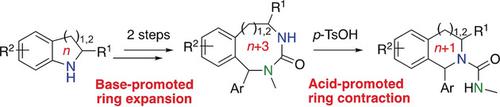
Tetrahydroisoquinolines and tetrahydrobenzazepines were prepared by acid‐promoted ring contraction of cyclic ureas, which were themselves formed by ring expansion of indolines and tetrahydroquinolines. The consequent overall one‐carbon insertion reaction gives these 6‐ and 7‐membered heterocyclic scaffolds in three steps from readily available precursors. Other ring sizes may be formed by an alternative elimination reaction of bicyclic structures. Scalability of the method was demonstrated by operating it in a flow system.
中文翻译:

从容易获得的杂环前体合成1-芳基四氢异喹啉和四氢苯并ze庚因的连续扩环和收缩
四氢异喹啉和四氢苯并ze庚因是通过酸促进环状脲的环收缩而制备的,环状脲本身是由二氢吲哚和四氢喹啉的环膨胀形成的。随之而来的整体一碳插入反应可通过三步法从易于获得的前体中获得这些6元和7元杂环骨架。其他环尺寸可以通过双环结构的替代消除反应形成。该方法的可扩展性通过在流动系统中进行操作来证明。
更新日期:2018-04-17
中文翻译:

从容易获得的杂环前体合成1-芳基四氢异喹啉和四氢苯并ze庚因的连续扩环和收缩
四氢异喹啉和四氢苯并ze庚因是通过酸促进环状脲的环收缩而制备的,环状脲本身是由二氢吲哚和四氢喹啉的环膨胀形成的。随之而来的整体一碳插入反应可通过三步法从易于获得的前体中获得这些6元和7元杂环骨架。其他环尺寸可以通过双环结构的替代消除反应形成。该方法的可扩展性通过在流动系统中进行操作来证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号